East Asian Arch Psychiatry 2017;27:142-9
ORIGINAL ARTICLE
Dr Antonios Paraschakis, MD, MSc, PhD, Psychiatric Hospital of Attica “Dafni”, Athens, Greece.
Dr Aristeidis H. Katsanos, MD, PhD, Department of Neurology, University of Ioannina School of Medicine, Ioannina, Greece.
Address for correspondence: Dr Antonios Paraschakis, MD, MSc, PhD, 95
Ioanninon Street, 16674 Glyfada, Attica, Greece.
Tel: (30) 2109636097 / (30) 6977706892; Fax: (30) 2132054300; Email: antparaschakis@yahoo.gr
Submitted: 11 October 2016; Accepted: 20 October 2017
Abstract
Introduction: Depression following traumatic brain injury is experienced by 16% to 60% of affected patients. The present study aimed to update the best evidence-based pharmacological treatments for tackling such chronic and debilitating disorders.
Methods: We systematically reviewed and meta-analysed randomised controlled trials published from 1990 until August 2017 that compared the efficacy of antidepressants with placebo in the treatment of post-traumatic brain injury depression. We searched MEDLINE, SCOPUS, and the Cochrane Central Register of Controlled Trials (CENTRAL).
Results: Four studies were eligible for the meta-analysis. The antidepressants studied were the selective serotonin reuptake inhibitors sertraline and citalopram. The rate of non-responders at the end of the follow- up period was lower in the treatment groups compared with placebo (odds ratio = 0.42, 95% confidence interval: 0.15-1.17); this difference was not statistically significant (p = 0.10). In subgroup analysis of the studies that reported mean Hamilton Depression Rating Scale score differences between treatment and control groups in both baseline and endpoint evaluations, the pooled mean difference was reduced from 2.11 (95% confidence interval: -1.25 to 5.46) to -2.36 (95% confidence interval: -5.59 to 0.87), in favour of the treatment group, though not statistically significant (p = 0.06). No evidence of heterogeneity was detected. In the subgroup analysis according to the antidepressant used in the included studies, there was a trend towards statistical significance for sertraline only (odds ratio = 0.28, 95% confidence interval: 0.08-1.03; p = 0.05); this was not evident in the study that reported the use of citalopram (odds ratio = 0.83; 95% confidence interval: 0.15-4.64; p = 0.84).
Conclusions: Sertraline might be effective, though not statistically significant, in treating patients with post-traumatic brain injury depression. Adequately powered randomised controlled trials — extended to the plethora of newer antidepressants aiming to prove their non-inferiority to the selective serotonin reuptake inhibitors studied — are needed to confirm our results. The dearth of quality studies of this devastating problem of public health is rather impressive.
Key words: Brain injuries, Traumatic; Depression
Introduction
Traumatic brain injury (TBI) is extremely common. In the US alone, more than 1,400,000 new cases of head trauma occur every year; over 3 million patients have TBI-related disabilities.1 The direct medical and indirect costs for head injuries for the year 2000 alone exceeded US$60 billion.1 The population of brain trauma patients is relatively young with a presumably long life expectancy.2 In fact, in individuals aged < 45 years, head trauma is the main cause of death and disability.3
The psychological difficulties that may arise after a brain trauma vary widely and are of diverse severity. They include mood disorders, generalised anxiety disorder, obsessive-compulsive disorder, panic disorder, and post- traumatic stress disorder.4 Approximately 40% of patients with TBI have at least 2 psychiatric conditions, with depressive disorders having the lion’s share among them — studies report rates between 16% and 60%.3,5 Major depression afflicts 24% to 35% of TBI patients, with a prevalence of 61% within the first 7 years post-trauma.3,6-8
The incidence of head trauma in men is apparently double that in women who nonetheless appear to have double the incidence of post-injury depressive disorders.9 Major and moderate depression, adjustment disorder with depressive symptoms, and dysthymia can persist for (or occur) a long time, often years, after the injury.10 Such disorders appear 142 Also, it appears that individuals with milder forms of head injury are at higher risk of depressive disorders.12-14
The impact of co-morbid depressive disorders on a patient’s quality of life can be severe, with difficulties returning to work as well as enjoying social activities and recreation time, sexual dysfunction, deterioration of general health, and increased caregiver burden.15,16 Depression can also delay the recovery of several post-trauma physical symptoms, such as headache, neck pain, blurred vision, sleep problems, fatigue and cognitive symptoms, and can lead to prolonged and ineffective hospital stays.1,17
Pharmacotherapy constitutes the mainstay of treatment for moderate and major depressive disorders following head injury. The present study systematically searched and meta-analysed the latest pertinent studies in an attempt to determine the best evidence-based antidepressant medication for post-TBI depressive disorders. We also highlighted the gaps in the literature and provided suggestions for further research.
Methods
Study Selection and Eligibility Criteria
This meta-analysis was presented according to the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines for systematic reviews and meta-analyses.18 Eligible placebo-controlled randomised controlled trials (RCTs) that compared antidepressants with placebo in adult patients with depressive disorders (major and moderate depression, adjustment disorder with depressive symptoms, dysthymic disorder) following TBI were identified in MEDLINE, SCOPUS, and the CENTRAL Register of Controlled Trials. The following keywords were used in all database searches: “antidepressants”, “traumatic brain injury”, “depressive disorders”, “depression”, “brain trauma”, “brain injury”, “head trauma”, “head injury”, “selective serotonin reuptake inhibitors”, “serotonin- norepinephrine reuptake inhibitors”, “serotonin modulators and stimulators”, “serotonin antagonists and reuptake inhibitors”, “norepinephrine reuptake inhibitors”, “tricyclic antidepressants”, “tetracyclic antidepressants”, and “monoamine oxidase inhibitors”. No language or other filters were imposed. Our search was restricted to the period from 1990 to 2017 with the last search performed on 6 August 2017. We also examined the references of all retrieved articles to identify studies that might have been missed by the initial database investigation.
The search was performed independently by 2 reviewers (authors) and included only placebo-controlled RCTs that reported changes in the Hamilton Depression Rating Scale (HAM-D) during the follow-up period. The following papers were excluded from further analysis: (1) observational studies; (2) case series or case reports; (3) clinical trials with no randomisation protocol; (4) RCTs without placebo subgroups; and (5) studies that reported primary outcome other than depression. Emerging disagreement regarding the literature search results between the 2 authors was resolved through consensus.
Quality Assessment
In each eligible study we used a pre-defined 7-point quality control (Cochrane Risk of Bias Tool) to address biases. For each quality item the corresponding risk of bias was categorised as low, high, or unclear according to the suggestions by Higgins et al.19 Complete outcome data were judged as ‘low risk’ when the percentage of participants lost to follow-up was lower than 5% and ‘high risk’ when the reported figure exceeded 20%, or if significant imbalances were observed in the dropout / loss to follow-up rates between the treatment and placebo subgroups. In studies that reported rates of loss to follow-up between 5% and 20%, the risk of attrition bias was categorised as ‘unclear’.20 In the ‘other bias’ category we included all other potential sources of bias. Bias identification within studies was independently performed by the 2 reviewers (authors). All emerging conflicts in quality control were resolved through consensus.
Outcome Measures
The mean differences in HAM-D score between treatment and control patients both at baseline and endpoint evaluations, together with the corresponding standard deviations, were extracted independently after bias identification by the authors. We also extracted the absolute number of patients who were reported as non-responders at the end of the follow-up period for each study protocol.
Statistical Analysis
We calculated odds ratios (ORs) in each study protocol to compare the lack of response rate in TBI patients with depressive symptoms treated with antidepressants and those receiving placebo. An OR of < 1 indicated that the treatment under investigation had a positive effect in the amelioration of depressive symptoms in patients with TBI compared with placebo. A random-effects model21 was used to calculate the pooled ORs. The equivalent z test was performed for each pooled OR and a p value of < 0.05 was considered statistically significant. After the overall analysis we performed an additional subgroup analysis according to the antidepressants used in the included studies.
Unadjusted mean differences between treatment and placebo subgroups for both the baseline and endpoint evaluations were pooled in 2 different subgroups. A mixed- effects model was used to calculate both the pooled point estimate in each subgroup and the overall estimates. According to the mixed-effects model, a random-effects model was first used to combine studies within each subgroup, and then a fixed-effect model was used to combine subgroups and estimate the overall effect. We assumed the study-to-study variance (tau-squared) to be the same for all subgroups. Tau-squared was first computed within subgroups and then pooled across subgroups.
We assessed heterogeneity between studies with the Cochran Q and I2 statistics. For the qualitative interpretation of heterogeneity, I2 values of at least 50% were considered to represent substantial heterogeneity while values of at least 75% indicated considerable heterogeneity (as per the Cochrane Handbook).22
All statistical analyses were conducted using Review Manager (RevMan) Version 5.3 (Copenhagen, Denmark: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014). This study was done in accordance with the principles outlined in the Declaration of Helsinki.
Results
Study Selection and Study Characteristics
Systematic search of MEDLINE and SCOPUS databases yielded 277 and 377 results, respectively. Subsequent search of the CENTRAL Register of Controlled Trials retrieved no additional RCTs. After removing duplicates, the titles and abstracts from the remaining 602 studies were screened and 9 potentially eligible studies for meta-analyses were retained. After retrieving the full-text version of these studies, 5 were excluded because they reported primary outcomes other than depression, were not randomised, or reported only placebo lead-in design.
In the final presentation of the literature search results, there was no conflict or disagreement between the 2 reviewers and the 4 studies that met the study’s protocol inclusion criteria were included both in the qualitative and quantitative synthesis (Fig 1). The characteristics of the included studies, comprising 181 patients (67.8% males and 32.2% females, mean age 39.1 years) are summarised in Table 1.2,5,23,24 Excluded studies with reasons for exclusion are shown in Table 2.25-29
Risk of Bias of Independent Studies
Risk of bias in the included studies is summarised in Figure 2. Random sequence generation and allocation concealment was adequately reported in 1 study only.2 In the other 3 trials,5,23,24 the risk of selection bias was considered high as they either reported selective and non-randomised selection of included patients from the initially recruited population5,23 or baseline characteristics were unbalanced between the 2 subgroups.24 Moreover, methods of allocation concealment were not reported in 3 trials.5,23,24 Blinding of participants, personnel and outcome assessment was sufficiently reported in 3 of 4 protocols.2,5,23 The risk of blinding on outcome assessment was considered high in 1 study protocol which reported that the former was broken at any time during the study if depression was confirmed.24
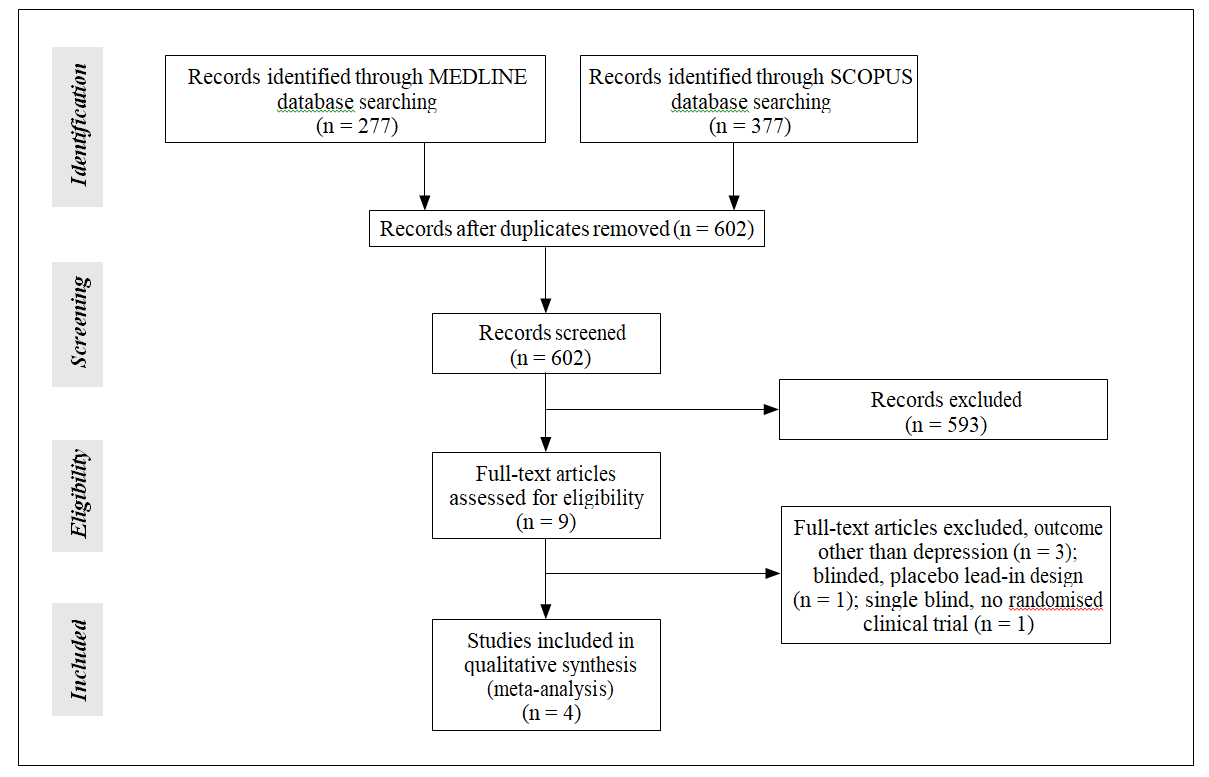
Figure 1. Flowchart of eligible studies. Table 1. Studies included in the meta-analysis.
Table 1. Studies included in the meta-analysis.
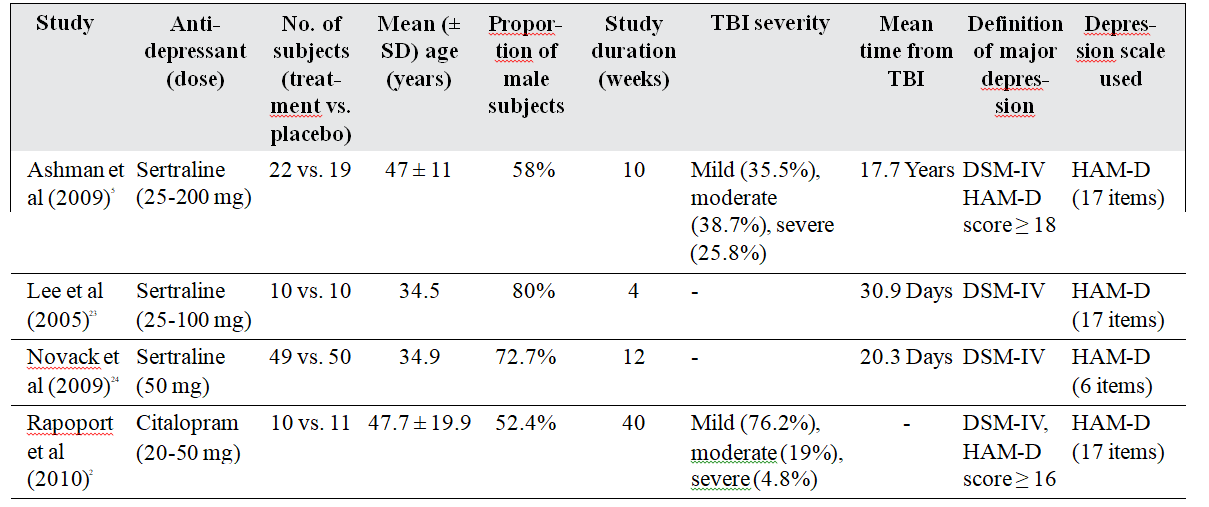
Abbreviations: HAM-D = Hamilton Depression Rating Scale; SD = standard deviation; TBI = traumatic brain injury.
Table 2. Excluded studies with reasons for exclusion.
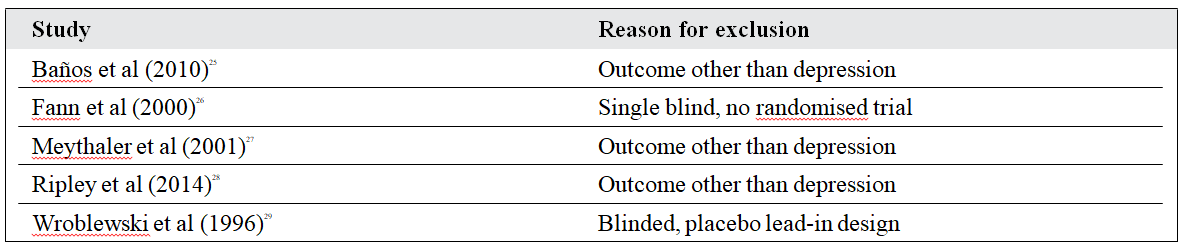
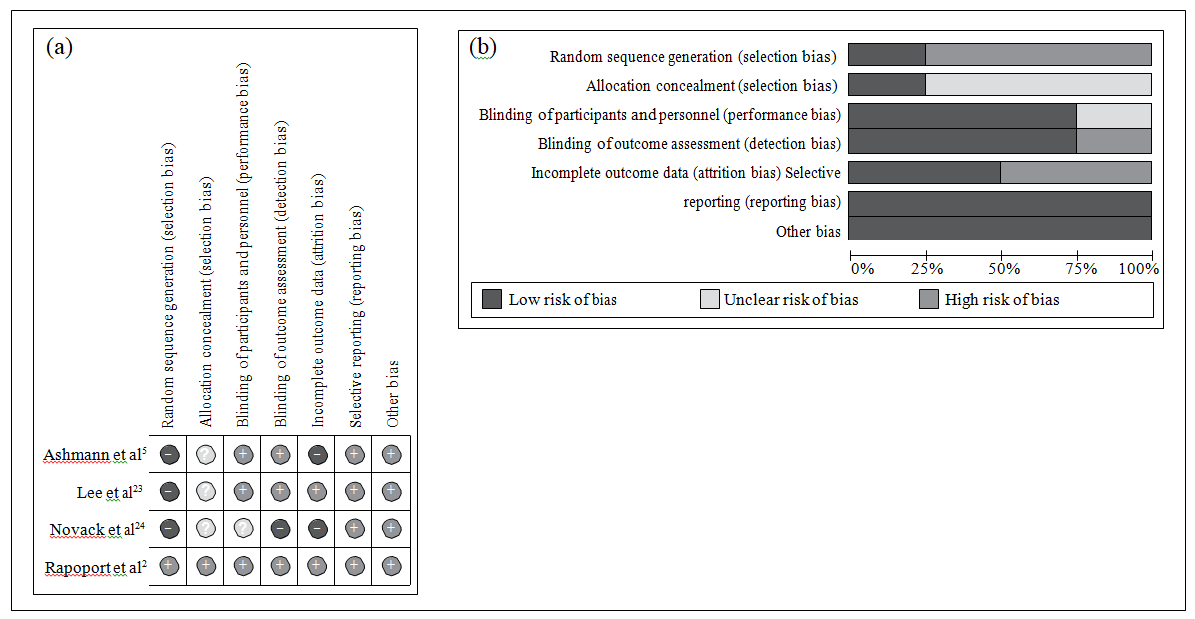
Figure 2. (a) Summary of authors’ judgements about each risk of bias item in the included studies and (b) graphs showing authors’ judgements about each risk of bias item presented as percentages across all included studies.2,5,23,24
One of the study protocols reported 22% of patients lost to follow-up,5 while another study not only reported high rates of loss but also differential dropout rates between groups.24 The risk of selective reporting and other biases was considered generally low in all included trials.
Overall Analysis and Subgroup Analyses
The rate of non-responders at the end of the follow-up period was lower in the treatment groups when compared with placebo (OR = 0.42, 95% confidence interval [CI]: 0.15- 1.17) [Fig 32,5,24], though this difference was not statistically significant (p = 0.10). There was no evidence of significant heterogeneity among the included studies (I2 = 0, p for Cochran Q: 0.39). In the subgroup analysis according to the antidepressant used in the included studies, even though statistical significance was not achieved for sertraline, there was a trend towards it (OR = 0.28, 95% CI: 0.08-1.03; p = 0.05); this association was not found in the study that used citalopram (OR = 0.83, 95% CI: 0.15-4.64; p = 0.84).
In subgroup analysis of the studies that reported mean HAM-D differences between treatment and control groups in both baseline and endpoint evaluations, the pooled mean difference was reduced from 2.11 (95% CI: -1.25 to 5.46) to -2.36 (95% CI: -5.59 to 0.87) in favour of the treatment group (Fig 45,23). Despite this reduction, statistical significance was not achieved (p = 0.06). No evidence of heterogeneity among the studies included in each subgroup was detected (I2 = 0 and p > 0.90 in both subgroups).
Discussion
Only 4 studies fulfilled the eligibility criteria of our search, and all had methodological shortcomings. The studies showed that treatment with the selective serotonin reuptake inhibitor (SSRI) sertraline is likely to be beneficial in treating post-TBI depressive disorders (there was a trend
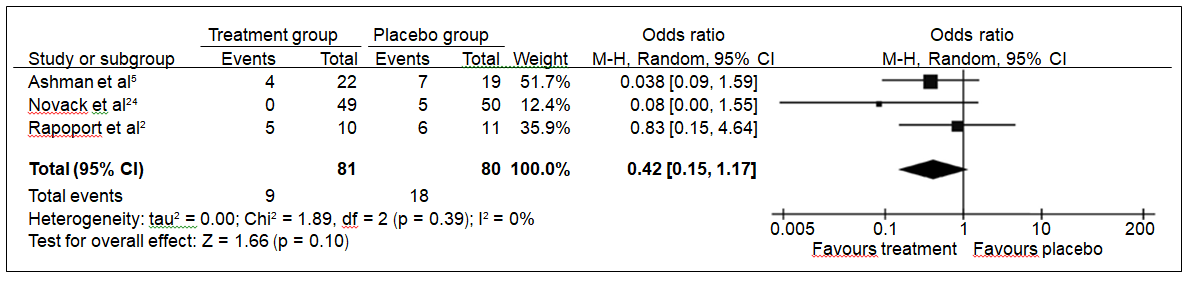
Figure 3. Overall analysis of rate of non-responders receiving treatment for post-TBI depression at the end of follow- up period.2,5,24
Abbreviations: CI = confidence interval; df = degrees of freedom; M-H = Mantel-Haenszel; TBI = traumatic brain injury.
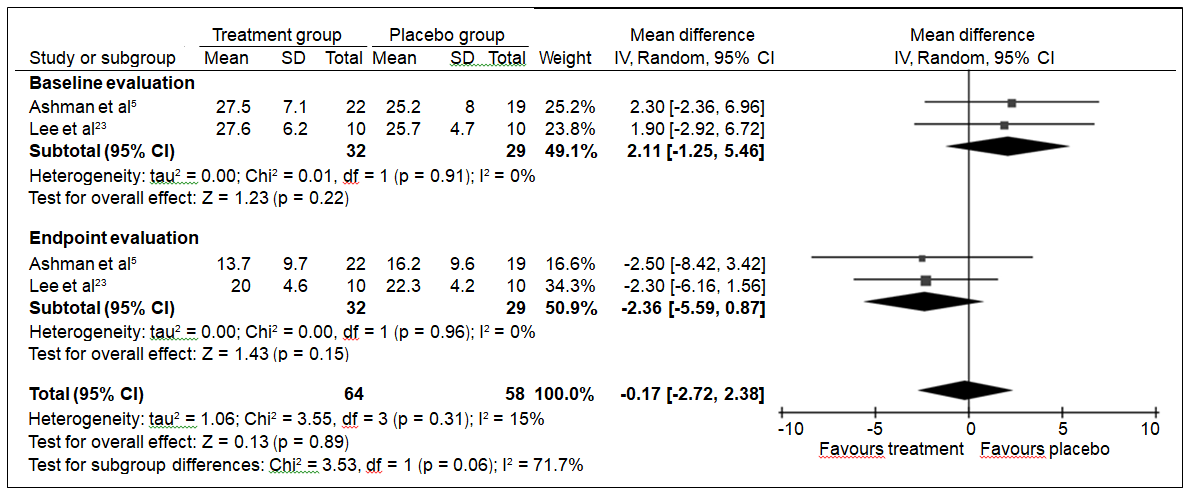
Figure 4. Subgroup analysis of studies reporting HAM-D scores in both baseline and endpoint evaluations.5,23
Abbreviations: CI = confidence interval; df = degrees of freedom; HAM-D = Hamilton Depression Rating Scale; SD = standard deviation.
towards significance). The particularly small number of included studies may explain this marginal result.
Sertraline has a favourable side-effect profile and can be titrated rapidly; this could explain why it has been preferred in the majority of RCTs involving such patients. A start-low go-slow titration scheme, with close monitoring for adverse effects (particularly common in this patient group, comprising seizures, sedation, drowsiness, and cognitive deterioration) is advised.1 Additionally, sertraline subtly inhibits dopamine reuptake — a valuable asset when a deficit in dopamine activity is present — as is often the case in brain injuries.30,31 Besides, the most plausible hypothesis for post-TBI depression supports the role of multiple neurotransmitter deficits and not a single one.23,32
Although serotonin can certainly improve mood regulation, impulse and anxiety in patients with post- TBI depression, noradrenaline may assist in ameliorating post–head trauma apathy, lack of motivation and energy.9 Therefore, there appears to be a need to study dual-action antidepressants such as serotonin and noradrenaline reuptake inhibitors (SNRIs) or mirtazapine for the treatment of post–brain trauma depressive disorders. Serotonin and noradrenaline reuptake inhibitors may also represent an option for post-trauma pain symptoms due to their analgesic effects.33 Finally, given that the co-morbidity of depressive and anxiety disorders after TBI appears common, varying between 11% and 77%, SNRIs merit an additional consideration given their indication for the treatment of similar conditions in non-TBI populations.5,34
Use of monoamine oxidase inhibitors is problematic due to dietetic restrictions, while tricyclic antidepressants appear to have a limited role in cases of post–head trauma depression because of their anticholinergic and pro- convulsive effects.3 Anticholinergic actions, in particular, may have a detrimental effect on the cognitive dysfunction frequently encountered in head injuries of medium or severe gravity.35 The noradrenaline and dopamine reuptake inhibitor bupropion seems to have a limited role in such patients given its seizure threshold lowering effect.36 Nevertheless others insist that this dose-related side-effect has been overemphasised.35
In our view, future research should shed light on whether there are high-risk subgroups within the population of patients with TBI and if there is a role for prophylactic pharmacological treatments that can restore or even reverse neurotransmitter dysfunction. Antidepressant pharmacotherapy in the treatment of patients with recurrent post-TBI depression may also warrant investigation. Data have shown that patients with brain trauma are vulnerable to recurrent depression regardless of antidepressant prophylaxis, are more susceptible to medication side- effects, and, seemingly, are more resistant to traditional pharmacological regimens.2,4,17 Whether particular symptoms of post-trauma depression are more or less responsive to antidepressant treatment may be a further area of study. The most appropriate combination with psychotherapy may represent another field of research given that combination strategies seem more effective in major depressive disorders.37
Our study was the first to meta-analyse randomised controlled studies of antidepressant pharmacotherapy for post-TBI depression since the one by Price et al38 in 2011 that nonetheless included various neurological disorders and was not strictly limited to post-TBI depression. There are only 2 systematic reviews on this subject1,39; comprehensive data were also provided by the qualitative reviews.4,35
The meta-analysis lends further support to the very recent expert consensus on drugs for behavioural disorders after TBI that recommends antidepressant treatment in patients with TBI and depression, with a higher standard of proof for SSRIs.39
Limitations
It should be noted that we performed no search of grey literature (i.e. literature that has not been formally published). Given that published trials tend to be larger and show an overall greater treatment effect than grey trials, and taking into account the small number of included studies, the possibility of publication bias in the present meta- analysis cannot be excluded.40,41 Additionally, the mean time interval after brain injury varied considerably among the studies and the duration of trials was relatively short. Trauma severity also varied, with mild and moderate cases being more common than severe ones. The area of brain injury was rarely specified, even though its impact on the occurrence of depression and the response to treatment may be significant. It should be noted that for each study protocol we included only RCTs that reported changes in the HAM-D during follow-up, a scale that is not specifically designed for patients with post-TBI depressive disorders (brain injury symptoms such as fatigue, apathy, frustration, or poor concentration may have overlapped with the symptoms of depression). Accordingly, the lack of other depression assessment scales should be considered a major limitation of the present meta-analysis. Likewise, it should also be highlighted that in all study protocols the diagnosis of depression was based on the DSM-IV criteria and / or increased score on the HAM-D.
Conclusions
The meta-analysis of antidepressants for the treatment of post-TBI depressive disorders showed that the SSRI sertraline might be effective, though not statistically significant. Nevertheless our results are based on very few and rather old studies that have methodological limitations and should not be considered as evidence-based treatment guidelines. Strong and updated support for the efficacy of antidepressant pharmacotherapy for post-TBI depression is lacking. Additional research with properly designed and adequately powered RCTs, including some of the newer antidepressants aiming to prove their non-inferiority to the SSRIs studied, seems necessary. The scarcity of properly designed studies on this topic, notwithstanding the plethora of new antidepressants discovered over the last 27 years, was a remarkable finding of our survey. After all, it is difficult to deny that patients with brain injuries are a population with numerous risk factors, significant co-morbidities, and complex medication regimens that deserve diverse and well-researched treatment options.
Declaration
All authors have disclosed no conflicts of interest.
References
- Fann JR, Hart T, Schomer KG. Treatment for depression after traumatic brain injury: A systematic review. J Neurotrauma 2009;26:2383-402.
- Rapoport MJ, Mitchell RA, McCullagh S, Herrmann N, Chan F, Kiss A, et al. A randomized controlled trial of antidepressant continuation for major depression following traumatic brain injury. J Clin Psychiatry 2010;71:1125-30.
- Vaishnavi S, Rao V, Fann JR. Neuropsychiatric problems after traumatic brain injury: unraveling the silent epidemic. Psychosomatics 2009;50:198-205.
- Neurobehavioral Guidelines Working Group; Warden DL, Gordon B, McAllister TW, Silver JM, Barth JT, Bruns J, et al. Guidelines for the pharmacological treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma 2006;23:1468-501.
- Ashman TA, Cantor JB, Gordon WA, Spielman L, Flanagan S, Ginsberg A, et al. A randomized controlled trial of sertraline for the treatment of depression in persons with traumatic brain injury. Arch Phys Med Rehabil 2009;90:733-40.
- Hibbard MR, Uysal S, Kepler K, Bogdany J, Silver J. Axis I psychopathology in individuals with traumatic brain injury. J Head Trauma Rehabil 1998;13:24-39.
- Holsinger T, Steffens DC, Phillips C, Helms MJ, Havlik RJ, Breitner JC, et al. Head injury in early adulthood and the lifetime risk of depression. Arch Gen Psychiatry 2002;59:17-22.
- Ashman TA, Spielman LA, Hibbard MR, Silver JM, Chandna T, Gordon WA. Psychiatric challenges in the first 6 years after traumatic brain injury: cross-sequential analyses of Axis I disorders. Arch Phys Med Rehabil 2004;85:S36-42.
- Kanetani K, Kimura M, Endo S. Therapeutic effects of milnacipran (serotonin noradrenalin reuptake inhibitor) on depression following mild and moderate traumatic brain injury. J Nippon Med Sch 2003;70:313-20.
- Karzmark P, Hall K, Englander J. Late-onset post-concussion symptoms after mild brain injury: the role of premorbid, injury-related, environmental, and personality factors. Brain Inj 1995;9:21-6.
- 1 Oquendo MA, Friedman JH, Grunebaum MF, Burke A, Silver JM, Mann JJ. Suicidal behavior and mild traumatic brain injury in major depression. J Nerv Ment Dis 2004;192:430-4.
- Glenn MB, O’Neil-Pirozzi T, Goldstein R, Burke D, Jacob L. Depression amongst outpatients with traumatic brain injury. Brain Inj 2001;15:811-8.
- Rapoport M, McCauley S, Levin H, Song J, Feinstein A. The role of injury severity in neurobehavioral outcome 3 months after traumatic brain injury. Neuropsychiatry Neuropsychol Behav Neurol 2002;15:123-32.
- Dikmen SS, Bombardier CH, Machamer JE, Fann JR, Temkin NR. Natural history of depression in traumatic brain injury. Arch Phys Med Rehabil 2004;85:1457-64.
- Breed ST, Flanagan SR, Watson KR. The relationship between age and the self-report of health symptoms in persons with traumatic brain injury. Arch Phys Med Rehabil 2004;85 Suppl 2:S61-S67.
- Jorge RE, Robinson RG, Moser D, Tateno A, Crespo-Facorro B, Arndt S. Major depression following traumatic brain injury. Arch Gen Psychiatry 2004;61:42-50.
- Rapoport MJ, Chan F, Lanctot K, Herrmann N, McCullagh S, Feinstein A. An open-label study of citalopram for major depression following traumatic brain injury. J Psychopharmacol 2008;22:860-4.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1-34.
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928.
- Sackett DL, Richardson WS, Rosenberg W, Hayes RB. Evidence- based medicine: How to practice and teach EBM. New York: Churchill Livingstone; 1997.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88.
- Deeks JJ, Higgins JP, Altman DG. Cochrane handbook for systematic reviews of interventions. Available from: http://handbook.cochrane.org/chapter_9/9_analysing_data_and_undertaking_meta_analyses.htm. Updated March 2011. Accessed 31 May 2017.
- Lee H, Kim SW, Kim JM, Shin IS, Yang SJ, Yoon JS. Comparing effects of methylphenidate, sertraline and placebo on neuropsychiatric sequelae in patients with traumatic brain injury. Hum Psychopharmacol 2005;20:97-104.
- Novack TA, Baños JH, Brunner R, Renfroe S, Meythaler JM. Impact of early administration of sertraline on depressive symptoms in the first year after traumatic brain injury. J Neurotrauma 2009;26:1921-8.
- Baños J, Novack T, Brunner R, Renfoe S, Lin HY, Meythaler J. Impact of early administration of sertraline on cognitive and behavioral recovery in the first year after moderate to severe traumatic brain injury. J Head Trauma Rehabil 2010;25:357-61.
- Fann JR, Uomoto JM, Katon WJ. Sertraline in the treatment of major depression following mild traumatic brain injury. J Neuropsychiatry Clin Neurosci 2000;12:226-32.
- Meythaler JM, Depalma L, Devivo MJ, Guin-Renfoe S, Novack TA. Sertraline to improve arousal and alertness in severe traumatic brain injury secondary to motor vehicle crashes. Brain Inj 2001;15:321-31.
- Ripley DL, Morey CE, Gerber D, Harrison-Felix C, Brenner LA, Pretz CR, et al. Atomoxetine for attention deficits following traumatic brain injury: results from a randomized controlled trial. Brain Inj 2014;28:1514-22.
- Wroblewski BA, Joseph AB, Cornblatt RR. Antidepressant pharmacotherapy and the treatment of depression in patients with severe traumatic brain injury: a controlled prospective study. J Clin Psychiatry 1996;57:582-7.
- Vecht CJ, van Woerkom CA, Teelken AW, Minderhoud JM. Homovanillic acid and 5-hydroxyindoleacetic acid cerebrospinal fluid levels: a study with and without probenecid administration of their relationship to the state of consciousness after head injury. Arch Neurol 1975;32:792-7.
- Stahl S. Classical antidepressants, serotonin selective and noradrenergic reuptake inhibitors. In: Stahl S, editor. Essential psychopharmacology. 2nd ed. San Diego, Cambridge University Press; 2006: 225-40.
- Arciniegas DB, Topkoff J, Silver JM. Neuropsychiatric aspects of traumatic brain injury. Curr Treat Options Neurol 2000;2:169-86.
- Stahl S. Pain and the treatment of fibromyalgia and functional somatic symptoms. In: Stahl S, editor. Stahl’s essential psychopharmacology. 3rd ed. San Diego, Cambridge University Press; 2008: 773-814.
- Baldwin DS. Serotonin noradrenaline reuptake inhibitors: A new generation of treatment for anxiety disorders. Int J Psychiatry Clin Pract 2006;10 Suppl 2:12-5.
- Alderfer BS, Arciniegas DB, Silver JM. Treatment of depression following traumatic brain injury. J Head Trauma Rehabil 2005;20:544- 62.
- Gartlehner G, Thieda P, Hansen RA, Gaynes BN, Deveaugh-Geiss A, Krebs EE, et al. Comparative risk for harms of second-generation antidepressants: a systematic review and meta-analysis. Drug Saf 2008;31:851-65.
- Shamsaei F, Rahimi A, Zarabian MK, Sedehi M. Efficacy of pharmacotherapy and cognitive therapy, alone and in combination in major depressive disorder. Hong Kong J Psychiatry 2008;18:76-80.
- Price A, Rayner L, Okon-Rocha E, Evans A, Valsraj K, Higginson IJ, et al. Antidepressants for the treatment of depression in neurological disorders: a systematic review and meta-analysis of randomised controlled trials. J Neurol Neurosurg Psychiatry 2011;82:914-23.
- Plantier D, Luauté J; SOFMER Group. Drugs for behavior disorders after traumatic brain injury. Systematic review and expert consensus leading to French recommendations for good practice. Ann Phys Rehabil Med 2016;59:42-57.
- Hopewell S, McDonald S, Clarke M, Egger M. Grey literature in meta- analyses of randomized trials of health care interventions. Cochrane Database Syst Rev 2007;(2):MR000010.
- Martin JL, Pérez V, Sacristán M, Alvarez E. Is grey literature essential for a better control of publication bias in psychiatry? An example from three meta-analyses of schizophrenia. Eur Psychiatry 2005;20:550-3.


