East Asian Arch Psychiatry 2017;27:115-20
ORIGINAL ARTICLE
Dr Tinarom Rungpetchwong, MD, Department of Psychiatry, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand.
Dr Surinporn Likhitsathian, MD, Department of Psychiatry, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand.
Mrs Siripan Jaranai, BSc (Psychiatric Nursing), Maharaj Nakorn Chiang Mai Hospital, Chiang Mai, Thailand.
Dr Manit Srisurapanont, MD, Department of Psychiatry, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand.
Address for correspondence: Dr Manit Srisurapanont, Department of Psychiatry, Faculty of Medicine, Chiang Mai University, 110 Intavaroros Road, Sri Phum, Muang, Chiang Mai 50200, Thailand.
Tel: (66-53) 935422; Fax: (66-53) 935426; Email: manit.s@cmu.ac.th
Submitted: 20 February 2017; Accepted: 25 July 2017
Abstract
Objective: To examine the distress related to individual depressive symptoms, the correlation between symptom distress and disability, and the gender difference in distress levels in patients with major depressive disorder.
Methods: This was a cross-sectional, observational study carried out at a university hospital providing tertiary care in northern Thailand. Participants were patients with major depressive disorder aged between 18 and 65 years. Depression severity was self-rated using the 9-item Patient Health Questionnaire (PHQ- 9). We expanded the 9 symptom items of the PHQ-9 into 13 individual symptoms. The participants rated their distress for each symptom on a scale of 0 to 4, from 0 indicating ‘not at all’ to 4 indicating ‘extremely’.
Results: A total of 130 (92 female and 38 male) patients with major depressive disorder participated in this study. Of the 13 symptoms, the distress level of overeating was lowest. Compared with overeating, the distress levels of feeling depressed / hopeless, feeling guilty, poor concentration, anhedonia, initial insomnia, middle / terminal insomnia, and fatigue were significantly higher and had a large effect size of differences (p < 0.001, Cohen’s dz ≥ 0.8). The distress levels related to feeling depressed / hopeless, feeling guilty, poor concentration, anhedonia, fatigue, suicidal ideation, and moving / speaking slowly were moderately and significantly correlated with overall functional impairment (Pearson’s r = 0.31-0.48, p < 0.001). Analysis of covariance, adjusted by the PHQ-9 total score, indicated no significant difference between men and women on any symptom.
Conclusions: Depressive symptoms related to high distress levels and moderately correlated with functional impairment were feeling depressed / hopeless, feeling guilty, poor concentration, and anhedonia.
Key words: Depressive disorder, major; Patient satisfaction; Sex factors
Introduction
Distress and disability related to a behavioural symptom or syndrome are key components of a mental state. Although emotional distress means mental suffering or anguish,1 disability is the limitation of certain functions in daily life due to an illness.2
In a similar way to disability (functional impairment or psychosocial dysfunction), distress is another generic criterion required for a diagnosis of mental disorders in the DSM-5.3 The ICD-10 views that distress or disability can be observed in most cases of mental disorder.4 The presence of clinically significant distress or disability also ensures that the disorder leads to significant consequences and that clinical treatment would be of help for the patient.
Understanding depressed patients’ view of distress and disability related to symptoms is beneficial for clinical practice and research. To coincide with patient concerns, priorities of treatment should be for symptoms that cause severe distress or disability. Along with the prevalence and severity of symptoms, research on the treatment of depression should focus on highly distressing and disabling symptoms. For example, highly distressing symptoms should be a target for the development of new antidepressants. In addition, the evidence in this area may be used as a guide for symptom-targeted therapies.
Little is known about the distress related to individual symptoms of depression. Based on the assumption that the depressive symptoms are equivalent and interchangeable,
few studies have examined the correlation between individual symptoms and distress or disability. Although some findings suggest a strong correlation between particular depressive symptoms and disability,5,6 few studies have examined distress related to depressive symptoms. A qualitative study showed that depressive symptoms can cause distress.7 Another recent study also reported the high distress levels of depressive, anxiety, and obsessive- compulsive syndromes in Asian patients with major depressive disorder (MDD).8 Nonetheless these studies did not examine the distress relative to individual symptoms of depression.
The lack of evidence of distress related to individual depressive symptoms may be partly due to the high level of subjectivity when determining distress levels and the difficulties in defining clinically significant distress.9 Most measures of depression, therefore, rarely assess the distress level associated with depression and its symptoms. For example, the Beck Depression Inventory I and II mainly assesses the frequency of and disability associated with depressive symptoms.10,11
Evidence suggests that gender differences in depression are real and not caused by methodological bias.12 Appetite and sleep abnormalities are more common in depressed women.13,14 It is not known if there are gender differences in the distress levels of depressive symptoms.
To the best of our knowledge, no study has examined the association between distress level and individual depressive symptoms. This study aimed to assess distress related to individual depressive symptoms in outpatients with MDD. This study was conducted to: (1) prioritise depressive symptoms based on the distress level perceived by patients; (2) examine the correlation between the distress level of each depressive symptom and the overall disability; and (3) determine the gender difference between distress levels associated with individual depressive symptoms.
Methods
Setting and Study Population
This was a cross-sectional observational study carried out between January 2016 and May 2016. The study population included Thai outpatients with MDD who sought treatment at Maharaj Nakorn Chiang Mai Hospital in northern Thailand. This tertiary care setting is a general hospital affiliated with Chiang Mai University. Participants gave written informed consent prior to taking part in the study following full explanation of procedures. The study was approved by the ethics committee for human research of Faculty of Medicine, Chiang Mai University.
We invited all outpatients of the psychiatric clinic who fulfilled the DSM-IV diagnosis of MDD and were aged between 18 and 65 years to enrol in the study. All other co- morbid physical and psychiatric conditions were permitted.
Assessment
The assessment was completed at a single visit. The study confirmed the presence of a current major depressive episode by the Mini-International Neuropsychiatric Interview (MINI), module A, major depressive episode.15
The participants rated their depression severity by using the 9-item Patient Health Questionnaire (PHQ-9).16 Based on the symptom frequency, each item of the PHQ-9 is rated 0 (not at all) to 3 (nearly every day). In addition, the level of PHQ-9 functional impairment was scored as follows: not difficult at all (0), somewhat difficult (1), very difficult (2), and extremely difficult (3).
Depressive symptoms listed for the enquiry of distress levels were drawn from those included in the PHQ-9. Three items of the PHQ-9 include ≥ 2 symptoms in an item. These are: (1) initial insomnia versus middle / terminal insomnia versus hypersomnia; (2) poor appetite versus overeating, and (3) moving / speaking slowly versus restlessness. To minimise the ambiguity of symptom interpretation, we separated the symptoms in these 3 items into 7 individual symptoms. A total of 13 depressive symptoms were therefore included in this study. These 13 symptoms were included in the questionnaire designed to assess distress related to individual symptoms of depression. The pattern of distress assessment applied in this questionnaire was drawn from the Symptom Checklist-90-R.17 Its key question was ‘How much were you distressed by…?’. The distress related to each individual symptom had 5 levels: not at all (0), a little bit (1), moderately (2), quite a bit (3), and extremely (4).
Analysis Plan
Of our study objectives, determining the gender difference between distress levels associated with individual depressive symptoms required the largest sample. Based on the record of our clinic, the proportion of male to female patients with MDD was 1 to 4. To achieve this, priority was given to obtaining an adequate sample of male patients. Based on the Cohen’s table of sample size calculation, we set the power and type I error (α) at 0.80 and 0.05, respectively. To detect the large effect size of mean differences in the distress levels between male and female patients, we needed at least 26 male patients. Based on the above-mentioned proportion of 1 to 4, the total sample of this study was set at 130 (26 males and 104 females).
Data were summarised as means (± standard deviations) and percentages. We used the Martinez-Iglewicz test to examine the normal distribution of continuous data.18
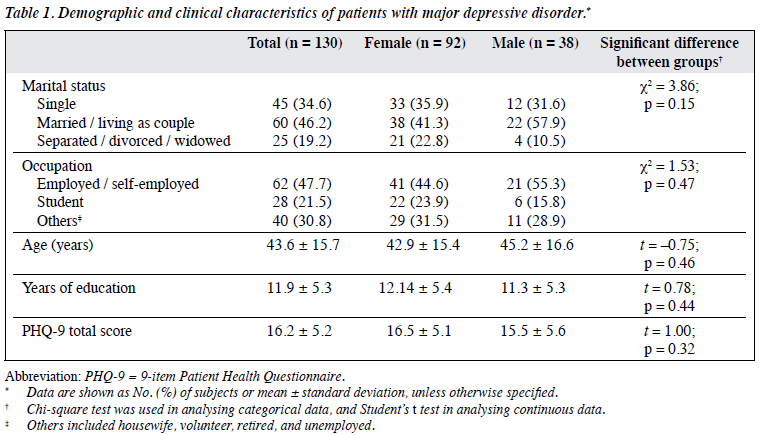
We determined the differences in socio-demographic and clinical characteristics between male and female participants by Chi-square and Student’s t tests.
We computed the mean and standard deviation of distress levels related to 13 individual symptoms. Paired t tests were used to examine the differences in distress levels between the symptom with lowest distress level and the other 12 symptoms. For each comparison, the ratio of t- statistic to the square root of the sample size was used to
19,20 determine the effect size of difference (Cohen’s dz). The Cohen’s dz was then classified as showing a small (0.2), medium (0.5), or large (0.8) effect.
The correlation between the distress level of each symptom and the overall functional difficulty of PHQ-9 was examined using the Pearson’s correlation coefficient r, which defined ≥ 0.70 as high correlation, 0.31 to 0.69 as moderate correlation, and ≤ 0.30 as low correlation.21
Because depression severity may affect distress levels, we conducted one-way analysis of covariance (ANCOVA) to determine a statistically significant gender difference for each individual symptom controlling for depression severity (PHQ-9 total score). For any significant difference after applying the ANCOVA, we further determined the magnitude (effect size) of the difference by calculating the ratio of sums of squares for whatever effect is of interest to the total sums of squares for all effects (η2).22 The η2 value was defined as showing a small (0.01), medium (0.06), or large (0.14) effect.19,20
All reported p values were 2-sided. To reduce the chances of obtaining false-positive results (type I errors), Bonferroni correction was used. By dividing the critical value of 0.05 by the number of testing, a p value of < 0.0042 (0.05/12) was used to indicate a significant difference of distress between symptoms. Similarly, a p value of < 0.0038 (0.05/13) was used to indicate a significant correlation of symptom with overall functional difficulty, as well as a significant gender difference in symptom distress. Apart from the Cohen’s dz and η2, which were computed using Excel 2013, data were also analysed using the NCSS 10 Statistical Software 2015 (LLC, Kaysville [UT], US).
Results
A total of 155 patients with MDD were invited to the study. By using the MINI, 18 female and 7 male patients were excluded because they did not meet the current diagnosis of major depressive episode.
This study included 130 (92 female and 38 male) patients with MDD who currently had a major depressive episode. Their mean age was 43.6 ± 15.7 years. The mean PHQ-9 total score of 16.2 ± 5.2 depicts the level of depression severity from moderately severe to severe. Table 1 presents the socio-demographic and clinical characteristics of the sample and shows no significant gender difference in any respect. For all continuous data of the whole sample, the Martinez-Iglewicz test did not reject the normal distribution of any data. We therefore used parametric tests for analysis of all continuous variables.
Of the 13 symptoms, overeating had the lowest mean distress level of 1.3 ± 1.3. Compared with overeating, the distress levels related to feeling depressed / hopeless, feeling guilty, poor concentration, anhedonia, initial insomnia, middle / terminal insomnia, and fatigue were significantly higher (p < 0.001) and had large effect sizes of differences (Cohen’s dz ≥ 0.08).
The distress levels related to feeling depressed / hopeless, feeling guilty, poor concentration, anhedonia, fatigue, suicidal ideation, and moving / speaking slowly were significantly and moderately correlated with overall functional impairment (r = 0.31-0.48, p < 0.001).
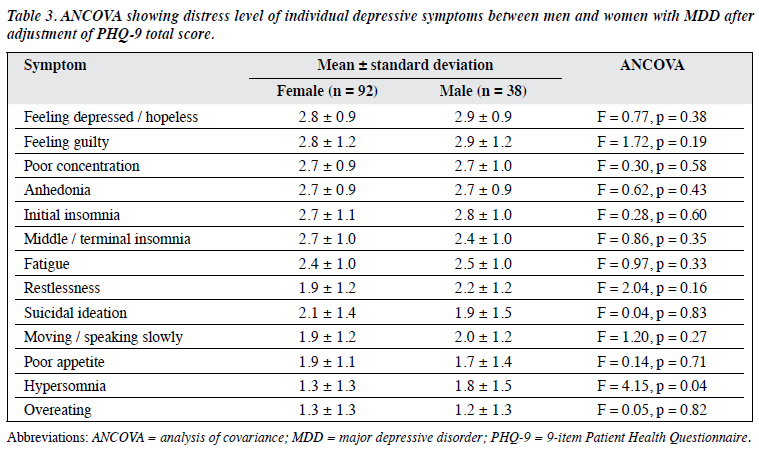
The ANCOVA analyses, adjusted using the PHQ-9 scores, found no significant gender difference in the distress levels for all symptoms (Table 3).
Discussion
This observational study was carried out in Thai outpatients with MDD who currently had moderately severe to severe depressive episodes. Highly distressed symptoms included feeling depressed / hopeless, feeling guilty, poor concentration, anhedonia, initial insomnia, middle / terminal insomnia, and fatigue. Feeling depressed / hopeless, feeling guilty, poor concentration, anhedonia, fatigue, suicidal ideation, and moving / speaking slowly were moderately correlated with overall functional impairment. Men and women appeared to have similar distress levels for all symptoms.
To the best of our knowledge, this is the first study to report the association of depressive symptoms with high distress levels. Surprisingly, the 7 symptoms that moderately correlated with overall functional impairment were the same as the first 7 symptoms related to functional impairment reported in a previous study.5 The replication of findings further supports the correlations between these 7 symptoms and functional impairment in depressed patients.
This study did not find any gender difference for the distress level of depressive symptoms. Although hypersomnia appeared to cause higher distress in men (p = 0.04), the significant difference was lost after adjustment for multiple testing.
The present findings will help healthcare professionals understand the patient’s view of their depressive symptoms. To coincide with patients’ concerns, priority of treatment should be given to those feeling depressed / hopeless, feeling guilty, poor concentration, anhedonia, initial insomnia, middle / terminal insomnia, and fatigue. Together with other aspects of a patient’s symptoms, e.g. severity, functional impairment and resistance to treatment, distress related to depression and its symptoms should also be considered when managing MDD. The correlation between symptom distress and disability suggests that patients with functional impairment are also likely to be distressed about their depression. Nonetheless, the correlation coefficients of ≤ 0.48 suggest that distress and disability are not highly correlated. These 2 aspects, therefore, are not interchangeable and cannot replace each other. Although the PHQ-9 may be able to identify depressed patients with functional impairment, due to its lack of distress assessment, it may overlook some patients with highly distressing depression.
There are limitations of this study. First, the small sample size, in particular the male group, may have produced false-negative findings in the comparison of distressing symptoms between men and women. Although this low-powered study appears to discover some true effects, e.g. significant differences or correlations, it is possible that the estimates of the magnitudes of these effects are exaggerated.23 Second, one should be careful in making generalisations from the present findings. Patients in other ethnic groups, especially those with different cultural backgrounds, may perceive or interpret each depressive symptom differently. The small number of male participants also limits the application of present findings in men with MDD. Third, this study examined only the depressive symptoms described in the DSM-5 and the PHQ-9. We decided to use PHQ-9 because it is a brief questionnaire that can assess all 9 symptoms of the DSM-5. Other common depressive symptoms, e.g. pessimistic thoughts, somatic symptoms that are included in some depression rating scales,24 were not considered. Although patients with MDD may have high anxiety syndrome,8 none of its symptoms were assessed in this study. In addition, without an objective rating scale of depression, it is difficult to determine the similarities and differences between the present sample and those in other studies. Fourth, actual disability was not assessed. It is possible that those with high distress were more pessimistic and thus tended to report more functional impairment. Fifth, physical and psychiatric co-morbidities that were not measured in this study might affect the study results, in particular the distress levels. By including participants with such co-morbidities, the present findings would be widely generalised. Lastly, due to the lack of aforementioned assessment, many factors that possibly confounded the results were not taken into account.
In conclusion, depressive symptoms are related to different levels of distress. Depressive symptoms related to high distress levels and moderately correlated with functional impairment included feeling depressed / hopeless, feeling guilty, poor concentration, and anhedonia. Distress levels related to depressive symptoms did not differ between men and women. Distress related to depression and its symptoms should be assessed and considered in managing MDD. Further studies with a larger sample size and in depressed patients with different cultural backgrounds are warranted.
Declaration
All authors have disclosed no conflicts of interest.
References
- Stedman TL. Stedman’s medical dictionary. 28th ed. Philadelphia: Lippincott Williams & Wilkins; 2006.
- Ustün B, Kennedy C. What is “functional impairment”? Disentangling disability from clinical significance. World Psychiatry 2009;8:82-5.
- Diagnostic and Statistical Manual of Mental Disorders, 5th ed (DSM- 5). Arlington, VA: American Psychiatric Association; 2013.
- International Statistical Classification of Diseases and Related Health Problems, 10th ed (ICD-10). Geneva: World Health Organization;1992.
- Fried EI, Nesse RM. The impact of individual depressive symptoms on impairment of psychosocial functioning. PLoS One 2014;9:e90311.
- Tweed DL. Depression-related impairment: estimating concurrent and lingering effects. Psychol Med 1993;23:373-86.
- Hanson B, Young MA. Why depressive symptoms cause distress: the clients’ perspective. J Clin Psychol 2012;68:860-74.
- Srisurapanont M, Hong JP, Tian-Mei S, Hatim A, Liu CY, Udomratn P, et al. Clinical features of depression in Asia: results of a large prospective, cross-sectional study. Asia Pac Psychiatry 2013;5:259- 67.
- Wheaton B. The twain meet: distress, disorder and the continuing conundrum of categories (comment on Horwitz). Health (London) 2007;11:303-19.
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 1961;4:561-71.
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory–II. San Antonio, TX: Psychological Corporation; 1996.
- Ustün TB. Cross-national epidemiology of depression and gender. J Gend Specif Med 2000;3:54-8.
- Parker G, Fletcher K, Paterson A, Anderson J, Hong M. Gender differences in depression severity and symptoms across depressive sub-types. J Affect Disord 2014;167:351-7.
- Silverstein B. Gender difference in the prevalence of clinical depression: the role played by depression associated with somatic symptoms. Am J Psychiatry 1999;156:480-2.
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998;59 Suppl 20:22-33.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-13.
- Derogatis LR. Symptom Checklist-90-R: Administration, scoring and procedures manual. 3rd ed. Minneapolis, MN: National Computer Systems Inc.; 1994.
- Martinez J, Iglewicz B. A test for departure from normality based on a biweight estimator of scale. Biometrika 1981;68:331-3.
- Cohen J. Statistical power analysis for the behavioral sciences. New York, NY: Routledge Academic; 1988.
- Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol 2013;4:863.
- Streiner DL. A checklist for evaluating the usefulness of rating scales. Can J Psychiatry 1993;38:140-8.
- Kennedy JJ. The eta coefficient in complex ANOVA designs. Educ Psychol Meas 1970;30:885-9.
- Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 2013;14:365-76.
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979;134:382-9.