Hong Kong J Psychiatry 2006;16:101-8
Original Article
Efficacy and Safety of Rivastigmine in the Treatment of Behavioural and Psychological Symptoms in Chinese Dementia Patients: an Open-Label Study
卡巴拉汀治療華裔痴呆症患者精神行為症狀的療效和安全性:開放研究
CF Chan, WC Chan, SW Li
陳暢輝、陳偉智、李兆華
Dr CF Chan, FHKAM (Psychiatry), Psychogeriatric Department, Castle Peak Hospital, Hong Kong, China.
Dr WC Chan, FHKAM (Psychiatry), Psychogeriatric Department, Castle Peak Hospital, Hong Kong, China.
Dr SW Li, FHKAM (Psychiatry), Psychogeriatric Department, Castle Peak Hospital, Hong Kong, China.
Tel: (852) 2456 7111; Fax: (852) 2463 1644; E-mail: chancf1@ha.org.hk
Submitted: 28 November 2006; Accepted: 30 January 2007
Abstract
Objective: Behavioural and psychological symptoms of dementia are integral features of Alzheimer’s disease. They have been recognised as important predictors for premature institutionalisation and constitute major caregiver burdens. This study examined the efficacy of rivastigmine in the treatment of behavioural and psychological symptoms of dementia in Chinese older adults suffering from mild-to-moderately severe Alzheimer’s disease, under conditions reflecting everyday clinical care. Rivastigmine tolerability and its effects on cognition and functional status were also assessed.
Patients and Methods:This was a 20-week prospective, open-label, single-centre study. Twenty four psychogeriatric outpatients, who fulfilled DSM-IV diagnosis criteria for Dementia of the Alzheimer’s Type and exhibited behavioural and psychological symptoms of dementia, were treated with flexible doses of rivastigmine. Clinical responses were evaluated using the Chinese version of the Neuropsychiatric Inventory (CNPI), the Cantonese version of the Mini-Mental State Examination (CMMSE), and Functional Assessment Staging (FAST).
Results: At week 20, the mean CNPI total score decreased by 19.5 (p < 0.001) with significant improvements regarding delusions, depression / dysphoria, apathy, disinhibition, irritability / lability, aberrant motor behaviour, and night-time behaviour disturbance. Clinically significant reductions in behavioural and psychological symptoms of dementia were observed in 20 (83%) of the patients. The percentage change in CNPI total score correlated with percentage change in CNPI caregiver distress score (Spearman’s rho = 0.8, p < 0.001), but not with the rivastigmine dosage (Spearman’s rho = 0.004, p = 0.99) or change in CMMSE score (Spearman’s rho = –0.175, p = 0.41). No significant changes in CMMSE and FAST scores were demonstrated. The mean ± SD daily dose of rivastigmine was 9 ± 3 mg. Rivastigmine was well-tolerated in general, though a significant mean weight loss of 2 kg (p < 0.001) was noted.
Conclusion: This study suggested that rivastigmine was well-tolerated and effective in improving behavioural and psychological symptoms of dementia in Chinese patients with mild-to-moderately severe Alzheimer’s disease. Its beneficial effect on behavioural and psychological symptoms of dementia was independent of dosage and cognitive response. More rigorous investigations are needed to confirm and clarify the potential therapeutic role of the drug in ameliorating behavioural and psychological symptoms of dementia.
Key words: Behavioural symptoms; Cholinesterase inhibitors; Alzheimer disease
摘要
目的:老年痴呆症患者必然具有痴呆症精神行為症狀,這些症狀是造成照顧者沉重壓力的主要原因,而醫學界則視這些症狀為患者要否提早入住安老機構的重要指標。本研究檢視在日常的護理環境裹,以卡巴拉汀治療輕度至中重度痴呆症華裔老年患者精神行為症狀的療效。研究也評估卡巴拉汀的耐受性以及它對患者認知和功能的影響。
患者與方法::本研究為期20週。屬前瞻性開放的單中心研究。研究對象共24名門診的老年精神病人,他們符合《精神疾病診斷與統計手冊》第四版說明的老年痴呆症診斷標準,也表現痴呆症精神行為的症狀,須服用不同份量的卡巴拉訂。臨床回應的評估採用了神經精神料問卷中文版(CNPI) 、粵語版簡易精神狀態檢查處表(CMMSE) 和功能評估量表(FAST)。
結果:: 在第20週,CNPI 總得分平均值下降了19.5 (p < 0.001) ,患者在以下各方面都有顯著改善: 妄想、抑鬱/情緒低落、情緒淡漠/冷漠、抑制解除、易怒/情緒激動、異常的動作行為,以及睡眠。臨床觀察可見20位患者(佔83%)的痴呆症精神行為症狀明顯減少。CNPI總得分的百分率改變與CNPI照顧者憂慮得分的百分率改變具有關連(Spearman’s rho =0.8, p < 0.001),但與卡巴拉汀用量(Spearman’s rho =0.004, p < 0.99) 和CMMSE 得分轉變(Spearman’s rho =0.175, p =0.41)均無關連。CMM8E和FAST的得分並無明顯變化。 卡巴拉汀日用量的平均值± 標準差為9±3毫克。雖然患者平均減重達2公斤(p< 0.001), 但基本上對卡巴拉汀的耐受程度相當高。
結論:本研究顯示,患者對卡巴拉汀的耐受程度高,而該種藥物亦能有效減少輕度至中重度痴呆症華裔老年患者的痴呆症精神行為症狀, 但其療效與用藥量和認知回應無關。我們認為須進一步研究以釐清和確定卡巴拉汀在減輕痴呆症精神行為症狀可能發揮的治療作用﹒
關鍵詞:行為症狀、膽鹼酯酶抑制劑、老年痴呆患
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disorder characterised by progressive decline in memory and cognitive function, loss of ability to cope with activities of daily living, and disturbances in behaviour and emotions.1,2
It is the commonest form of dementia, accounting for about two-thirds of all cases.3 Until recently, researchers have focused primarily on interventions aiming to maintain cognitive and functional abilities of AD patients. Behavioural and psychological symptoms of dementia (BPSD) have received much less attention. Nonetheless, BPSD are common among these patients,4,5 and are associated with aggravated carer burdens,6 increased cost of care,7,8 and premature institutionalisation.9
In the past decade, there has been a growth in the research of pharmacological therapies for BPSD. Among them, use of atypical antipsychotics provides evidence for efficacy, but their effects are only modest.10
Besides, recent report of an increased likelihood of serious cerebrovascular adverse events in elderly demented patients treated with such agents has renewed interest in alternative therapies.11 Evidence suggests that cholinergic deficit, while contributing to cognitive symptoms, is also implicated in the neuropsychiatric manifestations of AD.12-14 This makes cholinesterase inhibitors, which enhance cholinergic neurotransmission, potential candidate drugs for the treatment of BPSD.
All currently marketed cholinesterase inhibitors have been reported to be effective in reducing BPSD in AD patients. In a 30-week randomised, placebo-controlled trial, tacrine ameliorated delusions and pacing, whilst enhancing cooperation, in patients with mild-to-moderate AD.15
Donepezil therapy demonstrated significant improvements in agitation and aggression, in a 6-month placebo-controlled study involving nursing home AD patients.16 In another study, moderate-to-severe AD patients treated with donepezil appeared to improve in terms of mood symptoms, psychosis, and agitation.17 Galantamine therapy was also associated with reduced emergence of behavioural disturbances and improvement in existing behavioural problems in patients with mild-to-moderate AD.18,19
Rivastigmine is a pseudo-irreversible inhibitor of both acetylcholinesterase and butyrylcholinesterase, and acts preferentially in the cortex and hippocampus. Evidently it is efficacious in ameliorating cognitive decline and improving global functioning in patients with mild-to-moderately severe AD.20-23 Its lack of adverse pharmacodynamic drug interactions makes it particularly suitable for the elderly population, who commonly receive multiple medications.24
This feature may partly account for the rising number of studies conducted in developed countries investigating its behavioural effects. Recent clinical data suggest that rivastigmine, like other cholinesterase inhibitors, exhibits beneficial psychotropic effects in AD patients. In a 2- year study, it was found to reduce mood disturbances and hallucinations, and stabilise aggressiveness, activity disturbances, as well as paranoid and delusional symptoms in patients with mild-to-moderate AD.25 Trials involving nursing home AD patients also showed an association of rivastigmine treatment with improvement and delay in the development of behavioural disturbances,26 particularly delusions, hallucinations, anxiety, disinhibition, irritability, and aberrant motor behaviour.27
In Hong Kong, rivastigmine is licensed for the treatment of cognitive deficits in patients with mild-to- moderate AD. While the aforementioned evidence suggests that it is generally effective for the management of BPSD, data supporting its use in local Chinese AD patients are lacking. The objective of this study was to assess the efficacy of rivastigmine in the treatment of BPSD in Chinese patients with mild-to-moderately severe AD, under everyday clinical settings. The effects of rivastigmine on cognition, tolerability, and safety were also explored.
Patients and Methods
This was a 20-week prospective open-label study, conducted in accordance with Good Clinical Practice for Trials on Medicinal Products in the European Community and the Declaration of Helsinki. The Ethics Committee for the New Territories West Cluster Hospitals approved the study protocol and statement of informed consent.
Subjects
The study participants were Chinese older adults referred for assessment at Psychogeriatric Clinic of Tuen Mun Mental Health Centre from 1 October 2001 to 29 March 2002. As required by the inclusion criteria, all patients were aged 65 or above and met the DSM-IV criteria for Dementia of the Alzheimer’s Type.1 Participants eligible for study entry had mild-to-moderately severe AD; they scored ≥ 11 and ≤ 22 on the Cantonese version of the Mini-Mental State Examination (CMMSE).28 In addition, they had to exhibit at least one very frequent or severe BPSD, as revealed by the Chinese version of the Neuropsychiatric Inventory (CNPI).29 Finally, they had to have a caregiver, who could ensure the patient’s medication compliance and report on progress at scheduled assessments. Exclusion criteria included other types of dementia, cognitive impairment due to toxic / alcoholic / traumatic aetiologies, history of other neurodegenerative or neurological conditions, co-morbid psychiatric disorders, severe / unstable physical illnesses, treatment with psychotropic medications within 30 days prior to screening, and any history of allergy to cholinergic compounds.
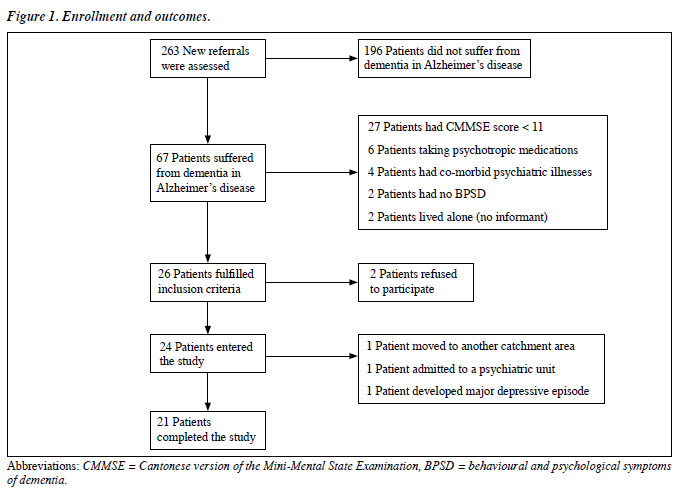
During the aforementioned period, 263 patients newly referred to the Psychogeriatric Clinic of Tuen Mun Mental Health Centre were assessed. Sixty seven patients fulfilled DSM-IV criteria for Dementia of the Alzheimer’s Type, of whom 27 had a CMMSE score of < 11, 6 were taking psychotropic medications, 4 had co-morbid psychiatric diagnoses, 2 exhibited no BPSD, 2 had no reliable informant available, and another 2 refused to participate. In the end, 24 patients were enrolled into this study (Figure 1).
Assessment Tools
The CNPI is a rater-administered, structured assessment tool for evaluation of BPSD. It assesses 12 neuropsychiatric symptom domains, namely delusions, hallucinations, agitation / aggression, depression / dysphoria, anxiety, euphoria, apathy, disinhibition, irritability / lability, aberrant motor behaviour, night-time behaviour disturbance, and changes in appetite and eating behaviour, based on the report of a caregiver who was considered to be a reasonably good observer of patient’s behaviour. The CNPI also assessed the distress of the caregiver (CNPI-D) in relation to each symptom domain.
The CMMSE is a fully structured scale for quantitative assessment of cognitive function. It consists of 11 items covering a multitude of relevant domains including orientation to time and place, registration and recall, attention and calculation, language abilities and visual construction. It has been widely used as a cognitive screening instrument and primary outcome measure for tracking progression of cognitive impairment in anti-dementia drug trials.
The Functional Assessment Staging (FAST) of dementia is a 16-item scale for gauging the functional deficits in AD patients,30 useful for staging the progression of dementia and monitoring the functional status of patients throughout the course of AD.
Study Design
This was a 20-week prospective open-label study. At the screening visit, informed consent, demographic data, medical and psychiatric history, and information about concomitant medications were obtained. Physical examination, blood tests (including complete blood picture, liver and renal function tests, thyroid function tests, syphilis serology, vitamin B12 level, red cell folate level, fasting sugar, fasting triglycerides and cholesterol), an electrocardiogram and computed tomography of the brain were performed.
During the baseline visit scheduled within 4 weeks of the screening visit, the patients were evaluated with the following efficacy measures: CNPI, CMMSE, and FAST. adverse event occurred, when consent was withdrawn, or if there was a change in residence. Evaluation of efficacy and tolerability was to be performed on dropout subjects within 2 working days of the final dose of rivastigmine.
Statistical Analysis
All recruited patients were included in the efficacy and tolerability analysis. For continuous measures, data from baseline, week 10, and week 20 assessments were analysed using the Friedman test. Individual comparisons between groups were made using Wilcoxon signed rank test (for paired data) or the Mann-Whitney U test (for unpaired data). Correlations between the percentage change of CNPI total score and rivastigmine dosage / percentage change of CNPI-D score / change in CMMSE score were explored using Spearman’s rho. For all analyses, a p value of less than 0.05 was considered statistically significant. Descriptive statistics were generated for demographic data, efficacy, safety and tolerability variables. Computations were performed using the Statistical Package for the Social Sciences version 10.0.
Results
Subject Characteristics
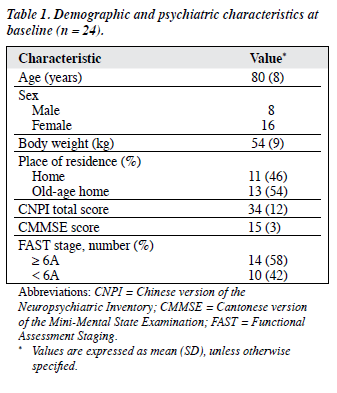
Demographic and clinical characteristics of participants at baseline are summarised in Table 1. The mean ± SD age of the patients was 80 ± 8 years. There was a preponderance of females in the recruited sample. Eleven (46%) of the patients resided at home.
At baseline, the mean ± SD CNPI total score was 34 ± 12. There was no statistically significant difference between the mean ± SD CNPI total scores of those living at home (33.2 ± 14.3) and in old-age homes (34.9 ± 11.0) [p = 0.07, Mann-Whitney U test]. Night-time behavioural disturbance was the most common BPSD, affecting 22 (92%) of the patients, followed by apathy (83%), depression / dysphoria (75%), delusions (71%), irritability / lability (58%), aberrant motor behaviour (58%), agitation / aggression (58%), changes in appetite and eating behaviour (54%), and hallucinations (46%). The mean CMMSE ± SD score was 15 ± 3. Fourteen (58%) of the patients were at FAST stage 6A or above. Medical conditions were reported by 17 (71%) the patients; the majority having fewer than 3 physical diagnoses.
Twenty one (88%) of the patients completed the trial; dropouts were all female and had baseline CNPI total scores comparable to study completers. Among the 3 dropouts, one moved to an old-age home in another catchment area, another displayed aggressive behaviour requiring inpatient treatment, and the third developed a major depressive episode. All dropout patients were included in the efficacy and tolerability analysis using the last-observation-carry- forward principle.
Rivastigmine Dosage
At the end of the trial, the mean daily dose of rivastigmine was 9 ± 3 mg. Ten (42%) of the patients were on 12 mg/day, 6 (25%) on 9 mg/day, 6 (25%) on 6 mg/day, and the remaining 2 (8%) received 3 mg/day. There was no correlation between the rivastigmine dosage and percentage change of total CNPI score (Spearman’s rho = 0.004, p = 0.99).
Efficacy Measures
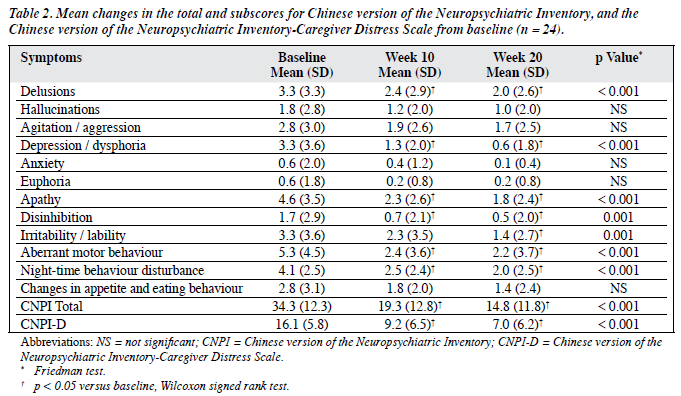
As depicted in Figure 2, there was a general trend towards a reduction in the number of patients exhibiting BPSD. However, significant reductions in the CNPI subscale score were observed only in the domains of delusions, depression / dysphoria, apathy, disinhibition, irritability / lability, aberrant motor behaviour, and night-time behaviour disturbance (Table 2). These reductions were evident at week 10 and maintained at week 20 (p < 0.05, Wilcoxon signed rank test), except for the irritability / lability subscore.
When compared to baseline, significant reduction in the mean CNPI total score was demonstrated at week 10 (-15.0, p < 0.05, Wilcoxon signed rank test) and week 20 (-19.5, p < 0.05, Wilcoxon signed rank test). At week 10, a reduction in the mean CNPI total score was observed in 22 (92%) of the patients; 16 of them achieving clinically significant (≥ 30%) reductions in the total CNPI score compared to baseline. At the end of the study, 4 more patients (i.e. 20 in total, 83%) showed clinically significant reductions in the total CNPI score.
Caregiver distress also improved over the study period. At baseline, the mean ± SD CNPI-D score was 16.1 ± 5.8 (Table 2). Statistically significant reductions were noted at weeks 10 and 20 (p < 0.05, Wilcoxon signed rank test). At the end of the study, 12 (50%) of the caregivers had ≥ 50% reductions in CNPI-D scores. Correlation was noted between the percentage change in the CNPI total score and the percentage change in CNPI-D score (Spearman’s rho = 0.8, p < 0.001).
Twelve (50%) of the patients had improved CMMSE scores at week 20 compared to baseline. The mean CMMSE score, however, did not change significantly throughout the study (p = 0.79, Friedman test). There was no correlation between the change in CMMSE score and the percentage change in CNPI total scores (Spearman’s rho = –0.175, p = 0.41). Nor was there a statistically significant change in mean FAST scores from baseline (p = 0.56, Friedman test).
Drug Safety and Tolerability
None of the patients withdrew from the study because of adverse events. At the end of the study, 10 (42%) were maintained on the maximum dosage of rivastigmine (12 mg/day). The most commonly reported adverse events were nausea, anorexia and dizziness, which affected 12 (50%), 11 (46%), and 9 (38%) of the patients respectively.
Other less frequently reported adverse events included malaise (21%), vomiting (21%), abdominal pain (8%), diarrhoea (8%), and headache (4%). Most of the symptoms were mild, and usually occurred during the first few weeks of dose titration.
No clinically significant changes in laboratory parameters, vital signs and electrocardiography findings were encountered. However, there were significant reductions in body weight (p < 0.001, Friedman test). At the end of the study, the mean weight loss from baseline was 2 kg. Five (21%) of the patients lost ≥ 7% of their body weight. There was no correlation between percentage body weight change and rivastigmine dosage (Spearman’s rho = 0.176, p = 0.41).
Discussion
This open-label study assessed the effect of rivastigmine on BPSD in Chinese patients with mild-to-moderately severe AD. The results indicate that rivastigmine was efficacious in alleviating BPSD. Statistically significant reductions were noted in a number of CNPI subscale scores, including delusions, depression / dysphoria, apathy, disinhibition, irritability / lability, aberrant motor behaviour, and night- time behaviour disturbance. This broad-spectrum BPSD response profile was similar to that reported by others.25-27
In the current study, 4 patients who did not respond to rivastigmine treatment at week 10 achieved clinically significant improvement at week 20, suggesting that in some patients it might take 3 to 5 months for the drug’s therapeutic effect on BPSD to become evident. Interestingly, behavioural improvement was shown to be independent of rivastigmine dosage and cognitive response, a feature not reported by others25-27 and for other cholinesterase inhibitors.15-19 Though BPSD had been identified as a major source of caregiver burden,6 few published trials commented on the effect of BPSD improvement on caregiver distress. In the current trial, reduction of caregiver distress, as measured by the CNPI-D score, was found to correlate significantly with improvement in BPSD.
Our study reported a favourable tolerability and safety profile for rivastigmine. The mean dosage of 9 ± 3 mg/day was comparable to therapeutic dosages identified in previous clinical trials.20-23 A total of 83% of the patients reported at least one treatment-related adverse event. But these were generally mild, occurred during dose escalation, and usually resolved with dose reduction. None of the participants withdrew from the study because of adverse events. High discontinuation rates reported in other published trials20-23,25-27 were probably due to too rapid forced dose escalation. The 2-week-interval flexible titration schedule employed in this study might have helped to minimise the dropout rate. No serious adverse events were reported throughout the study. Though there were no clinically significant changes in laboratory parameters, vital signs and electrocardiographic findings, a significant decrease in mean body weight of 2 kg was demonstrated at the end of the study; ≥ 7% weight loss being observed in 21% of the patients, comparable to reports in other trials.20-23,25-27
One of the strengths of the current study was that it resembled everyday clinical practice in Hong Kong, both in terms of the prescription of cholinesterase inhibitors and clinical characteristics of patients. The inclusion and exclusion criteria employed were largely consistent with local prescription guidelines for cholinesterase inhibitors. The baseline BPSD profiles of our study participants were also similar to those of other local psychogeriatric clinic popu- lations.4 However, the severity of BPSD at presentation was much worse than that reported in overseas studies.16-19,25-27
This might be due to the fact that Chinese demented patients tend to present to psychogeriatric clinic at later stages of the illness, with more severe BPSD.31 To minimise the effect of factors that were known to affect the presentation of BPSD, use of psychotropic medications was strictly prohibited prior to, and during, the present study. Patients were discontinued from the study if they developed co-morbid psychiatric disorders, the caregiver changed, or there was a change in the living environment. Such criteria were often not required or specified in previous published trials.
In the present study, the small number of subjects was a limitation. Collaboration with other centres or extending the duration of sampling period might have achieved a larger sample size. Another limitation was its open-label design, which was inherently associated with a multitude of selection and observation biases, and thus prone to false positive findings. Due to the absence of a control group for comparison, the drop in CNPI scores we encountered might in fact have been naturalistic, rather than attributable to a genuine behavioural effect of rivastigmine. Despite these shortcomings, an open-label design was employed because of ethical considerations. In a study by Fawlow et al23, AD patients were initially randomised to receive treatment with rivastigmine or placebo for 26 weeks, followed by a 26- week open-label extension phase, during which all subjects received rivastigmine treatment. At the end of this study (week 52), improvement in cognitive function in the control group failed to catch up with the treatment group, even after switching to rivastigmine during the open-label extension phase. This finding strongly suggested that rivastigmine did possess disease-modifying properties, and that delayed treatment could worsen outcome. Hence for the current study, placing patients eligible for rivastigmine treatment in the placebo group, and delaying active treatment for 20 weeks, was considered impractical. One last limitation of this study was that the p values were not adjusted for multiple comparisons, leading to increased probability of a type I error (i.e. false positive result).
In summary, this small-scale, open-label study demonstrates that rivastigmine is well-tolerated and effective in improving a broad spectrum of BPSD in Chinese patients with mild-to-moderately severe AD, under practical clinical conditions. This drug’s therapeutic effect is comparable to that of antipsychotic medications commonly used for treating BPSD, with the additional benefits of stabilising cognitive function and improving global functioning. Rivastigmine appears to be a promising new class of psychotropic agents for the management of BPSD in AD patients. More rigorous investigations employing robust methodologies entailing crossovers, and randomised, double-blind, placebo-controlled trials are needed to confirm and clarify its potential behavioural effects. Such measures could provide a better profile of its efficacy, ability to delay or reduce the emergence of BPSD in asymptomatic patients, long-term efficacy, core symptom profiles and predictors of response.
Declaration of Interest
This study was not supported by sponsorship or a grant from any organisation.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th edition. Washington, DC: American Psychiatric Press; 1994.
- Tatsch MF, Bottino CM, Azevedo D, Hototian SR, Moscoso MA, Folquitto JC, et al. Neuropsychiatric symptoms in Alzheimer’s disease and cognitively impaired, nondemented elderly from a community- based sample in Brazil: prevalence and relationship with dementia severity. Am J Geriatr Psychiatry 2006;14:438-45.
- Chiu HF, Lam LC, Chi I, Leung T, Li SW, Law WT, et al. Prevalence of dementia in Chinese elderly in Hong Kong. Neurology 1998;50:1002-9.
- Lam LCW, Tang WK, Leung VPY, Chiu HFK. Behavioural profile of Alzheimer’s disease in Chinese elderly – a validation study of the Chinese version of the Alzheimer’s disease behavioural pathology rating scale. Int J Geriatr Psychiatry 2001;16:368-73.
- Choy CN, Lam LC, Chan WC, Li SW, Chiu HF. Agitation in Chinese elderly: validation of the Chinese version of Cohen-Mansfield Agitation Inventory. Int Psychogeriatr 2001;13:325-35.
- Black W, Almeida OP. A systematic review of the association between the Behavioral and Psychological Symptoms of Dementia and burden of care. Int Psychogeriatr 2004;16:295-315.
- Zhu CW, Scarmeas N, Torgan R, Albert M, Brandt J, Blacker D, et al. Longitudinal study of effects of patient characteristics on direct costs in Alzheimer disease. Neurology 2006;67:998-1005.
- Herrmann N, Lanctôt KL, Sambrook R, Lesnikova N, Hébert R, McCracken P, et al. The contribution of neuropsychiatric symptoms to the cost of dementia care. Int J Geriatr Psychiatry 2006;21:972-6.
- Bianchetti A, Scuratti A, Zanetti O, Binetti G, Frisoni GB, Magni E, et al. Predictors of mortality and institutionalization in Alzheimer disease patients 1 year after discharge from an Alzheimer dementia unit. Dementia 1995;6:108-12.
- Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA 2005;293:596-608.
- Bullock R. Treatment of behavioural and psychiatric symptoms in dementia: implications of recent safety warnings. Curr Med Res Opin 2005;21:1-10.
- Cummings JL, Back C. The cholinergic hypothesis of neuropsychiatric symptoms in Alzheimer’s disease. Am J Geriatr Psychiatry 1998;6(2 Suppl 1):S64-78.
- Minger SL, Esiri MM, McDonald B, Keene J, Carter J, Hope T, et al. Cholinergic deficits contribute to behavioral disturbance in patients with dementia. Neurology 2000;55:1460-7.
- Lanari A, Amenta F, Silvestrelli G, Tomassoni D, Parnetti L. Neurotransmitter deficits in behavioural and psychological symptoms of Alzheimer’s disease. Mech Ageing Dev 2006;127:158-65.
- Raskind MA, Sadowsky CH, Sigmund WR, Beitler PJ, Auster SB. Effect of tacrine on language, praxis, and noncognitive behavioral problems in Alzheimer disease. Arch Neuro 1997;54:836-40.
- Tariot PN, Cummings JL, Katz IR, Mintzer J, Perdomo CA, Schwam EM, et al. A randomized, double-blind, placebo-controlled study of the efficacy and safety of donepezil in patients with Alzheimer’s disease in the nursing home setting. J Am Geriatr Soc 2001;49:1590-9.
- Gauthier S, Feldman H, Hecker J, Vellas B, Ames D, Subbiah P, et al. Efficacy of donepezil on behavioral symptoms in patients with moderate to severe Alzheimer’s disease. Int Psychogeriatr 2002;14:389-404.
- Tariot PN, Solomon PR, Morris JC, Kershaw P, Lilienfeld S, Ding C. A 5-month, randomised, placebo-controlled trial of galantamine in Alzheimer’s Disease. Neurology 2000;27:2269-76.
- Cummings JL, Schneider L, Tariot PN, Kershaw PR, Yuan W. Reduction of behavioral disturbances and caregiver distress by galantamine in patients with Alzheimer’s disease. Am J Psychiatry 2004;161:532-8.
- Agid Y, Dubois B. Efficacy and tolerability of rivastigmine in patients with dementia of the Alzheime type. Curr Ther Res 1998;59:837-45.
- Corey-Bloom J, Anand R, Veach J, ENA 713 B352 Study Group. A randomized trial evaluating the efficacy and safety of ENA 713 (rivastigmine tartrate), a new acetylcholinesterase inhibitor, in patients with mild to moderately severe Alzheimer’s disease. Int J Ger Psychopharmacol 1998;1:55-65.
- Rosler M, Anand R, Cicin-Sain A, Gauthier S, Agid Y, Dal-Bianco P, et al. Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: international randomised controlled trial. BMJ 1999;318:633-8.
- Farlow M, Anand R, Messina J, Hartman R, Veach J. A 52-week study of the efficacy of rivastigmine in patients with mild to moderately severe Alzheimer’s disease. Eur Neurol 2000;44:236-41.
- Grossberg GT, Stahelin HB, Messina JC, Anand R, Veach J. Lack of adverse pharmacodynamic drug interactions with rivastigmine and twenty-two classes of medications. Int J Geriatr Psychiatry 2000;15:242-7.
- Rosler M, Retz W, Retz-Junginger P, Dennler HJ. Effects of two-year treatment with the cholinesterase inhibitor rivastigmine on behavioural symptoms in Alzheimer’s disease. Behav Neurol 1999;11:211-6.
- Bullock R, Moulias R, Steinwachs KC, Cicin-Sain A, Spiegel R. Effects of rivastigmine on behavioral symptoms in nursing home patients with Alzheimer’s disease. International Psychogeriatrics 2001;13(Supp2):242.
- Cummings JL, Koumaras B, Chen M, Mirski D; Rivastigmine Nursing Home Study Team. Effects of rivastigmine treatment on the neuropsychiatric and behavioral disturbances of nursing home residents with moderate to severe probable Alzheimer’s disease: a 26-week, multicenter, open-label study. Am J Geriatr Pharmacother 2005;3:137-48.
- Chiu HF, Lee HC, Chung WS, Kwong PK. Reliability and validity of the Cantonese version of mini-mental state examination – a preliminary study. Journal of Hong Kong College of Psychiatrists 1994;4(sp2):25- 8.
- Leung VP, Lam LC, Chiu HF, Cummings JL, Chen QL. Validation study of the Chinese version of the neuropsychiatric inventory (CNPI). Int J Geriatr Psychiatry 2001;16:789-93.
- Reisberg B. Functional assessment staging (FAST). Psychopharmacol Bull 1988;24:653-9.
- Chow TW, Liu CK, Fuh JL, Leung VP, Tai CT, Chen LW, et al. Neuropsychiatric symptoms of Alzheimer’s disease differ in Chinese and American patients. Int J Geriatr Psychiatry 2002;17:22-8.