East Asian Arch Psychiatry 2015;25:146-149
Dr Mimi M. C. Wong, MBBS, MRCPsych, FHKAM (Psychiatry), FHKCPsych, Department of Psychiatry, United Christian Hospital, Kwun Tong, Hong Kong SAR, China.
Dr C. F. Chan, MBChB, MRCPsych, FHKAM (Psychiatry), FHKCPsych, Castle Peak Hospital, 1/F, Block F, Tuen Mun, Hong Kong SAR, China.
Dr S. W. Li, MBBS, MRCPsych, FHKAM (Psychiatry), FHKCPsych, Castle Peak Hospital, 1/F, Block F, Tuen Mun, Hong Kong SAR, China.
Ms Y. M. Lau (Diploma in RMN, MBA [HCA]), Castle Peak Hospital, 1/F, Block F, Tuen Mun, Hong Kong SAR, China.
Address for correspondence:
Tel: (852) 2379 9611; Fax: (852) 2717 1394; Email: wmc009@ha.org.hk
Submitted: 8 May 2015; Accepted: 9 July 2015
Abstract
Objective: To assess cognitive performance in elderly depressed patients following treatment for 6 months. Remission rate of depression after 6 months of treatment was calculated.
Methods: The study was performed in a consecutive group of patients aged ≥ 65 years with late-onset depression. Severity of depression was assessed by the Hamilton Depression Scale, cognitive performance by the Hong Kong Montreal Cognitive Assessment, and functional level by the Instrumental Activities of Daily Living Scale.
Results: A total of 52 patients were recruited. In all, 28 (53.8%) were found to have cognitive impairment at baseline and 8 (28.6%) of them had improvement after 6 months. This cognitively impaired group was older and had a lower Instrumental Activities of Daily Living Scale score. The remission rate of depression was 61.5%.
Conclusions: Cognitive impairment constituted a stable feature in a considerable number of elderly patients with depression. About two-thirds of patients achieved remission of depression after 6 months of treatment.
Key words: Age of onset; Depression; Mild cognitive impairment
Introduction
The prevalence of late-onset depression is estimated to be 15% among people aged ≥ 65 years, making it an important mental illness in the elderly population.1 It often arises in the context of medical and neurological disorders.2 It is frequently associated with cognitive impairments, suggesting a close relationship between the two.3,4 Cognitive impairment has been considered an accompanying symptom of depression and presents a clinical picture that resembles dementia (pseudodementia).5 Nonetheless it is now more commonly believed that depression may be an early manifestation of dementia.6 Early depressive symptoms among cognitively impaired individuals may represent an important preclinical sign, and confers an increased risk for impending Alzheimer’s disease or vascular dementia among old people.7-9 Their high co-morbidity may also be attributed to risk factors common for both disorders, mainly vascular risk factors.10
There is increasing evidence that the cognitive impairment in elderly depressed patients is irreversible since recovery from depression is generally not accompanied by improvement in cognitive performance.11,12 Lack of improvement in cognition following treatment for depression may be associated with an increased risk of later dementia.13 Self-reported decline in functional activities may be a marker for persistent cognitive impairment.12 Nonetheless cognitive impairment has no negative impact on recovery from depression.11,14
One of the objectives of this study was to assess cognitive performance in elderly depressed patients after they had received 6 months’ treatment for depression. We hypothesised that cognitive impairment at baseline would persist at follow-up despite improvement in depression. We also hypothesised that individuals with persistent cognitive impairment at 6 months had greater functional impairment at baseline. We also aimed to determine the remission rate of depression after 6 months of treatment.
Methods
The study was performed in a consecutive group of patients aged ≥ 65 years with depression who presented to the fast track clinic (FTC) of the Elderly Suicide Prevention Programme (ESPP) of Castle Peak Hospital, Hong Kong. The ESPP was founded in Hong Kong in 2002 with an aim of early detection of elderly at risk of suicide and to provide effective and adequate management to this group of patients. Castle Peak Hospital is 1 of 7 centres with an ESPP that caters to all referrals of elderly with suspected depression with or without suicidal attempt and who live in the New Territories West Cluster of Hong Kong where the population is about 1.1 million.
Inclusion criteria of the study were a diagnosis of depressive disorder (according to the ICD-10 criteria) and an onset of depression at age ≥ 50 years. Patients were excluded if they had other psychiatric diagnoses such as organic disorder, e.g. dementia, psychotic disorder, or a history of alcoholism or neurological disease.
Demographic data collected included age, gender, marital status, living status, education level, physical health status, and the presence of vascular risk factors. Vascular risk factors, including hypertension, diabetes mellitus, hyperlipidaemia, heart disease and cerebrovascular accident / transient ischaemic attack, were recorded. The number of risk factors was added to give a combined total score for vascular risk burden.
Assessment
The diagnoses of patients were made after clinical assessment by the case doctor and multidisciplinary case discussion chaired by an Associate Consultant of ESPP. Severity of depression was assessed using the Hamilton Depression Scale (HMD),15 cognitive performance by the Hong Kong Montreal Cognitive Assessment (HK-MoCA),16 and functional level by the Instrumental Activities of Daily Living Scale (IADL).17 The HK-MoCA is a useful cognitive screening instrument for use in older Chinese adults. The cutoff score for diagnosis of mild cognitive impairment was 21/22 with a sensitivity of 0.828, specificity of 0.735, and area under the curve of 0.920.18,19 It was found to have high test-retest, inter-rater reliability, and internal consistency.19 Its clinical utility in Chinese elderly subjects with predominately low education is generally good.16 The HK-MoCA and HMD were performed at baseline and repeated 6 months after presentation to the FTC. Patients were treated according to the management plan of their respective case doctors.
Data Analysis
Data were analysed using the Statistical Package for the Social Sciences Windows version 16.0 (SPSS Inc., Chicago [IL], US). For continuous data, variables were presented as mean and standard deviation for normally distributed data or as median and interquartile range (IQR) for skewed data. The Kolmogorov-Smirnov test was used to test for normality. For categorical data, variables were presented as numbers and percentages. The Mann-Whitney U test was used to compare continuous variables with skewed distribution if there were only 2 groups. For comparison of categorical data, the Pearson’s Chi-square or Fisher’s exact test was used. Spearman’s rank correlation coefficient was used to test for correlation of continuous variables with skewed distribution. The course of affective symptoms and cognitive performance was studied by means of paired Student’s t tests. Patient groups, such as cognitively impaired versus cognitively unimpaired, were compared using unpaired t tests or Chi-square tests.
Results
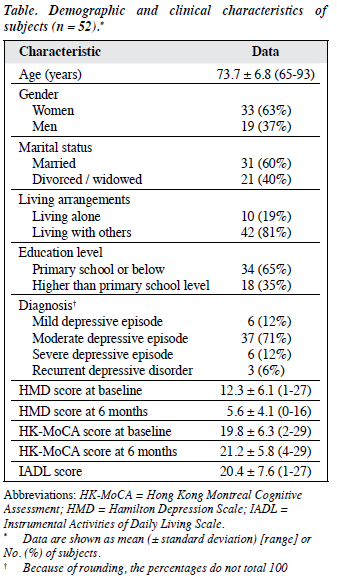
A total of 57 patients fulfilled the inclusion criteria but 5 were excluded as they were also diagnosed to have dementia; 52 patients were recruited. The demographic characteristics are summarised in the Table. In all, 47 (90.4%) were treated with antidepressants that included serotonin selective reuptake inhibitor, serotonin-noradrenaline reuptake inhibitor, noradrenergic and specific serotonergic antidepressant, and serotonin antagonist and reuptake inhibitor. Benzodiazepine was prescribed in 15 (28.8%) patients and hypnotics in 14 (26.9%). The number of vascular risk factors ranged from 0 to 4 (median, 1.29; IQR, 1.2). The difference in HMD and HK-MoCA scores at baseline and at 6 months was significant (p < 0.001, t test).
Course of Cognitive Impairment
In all, 28 (53.8%) patients were found to have cognitive impairment (HK-MoCA score < 22) at baseline. No significant difference was found in the demographic and clinical characteristics for those with or without cognitive impairment. Eight (28.6%) demonstrated improvement in their HK-MoCA score which returned to normal level after 6 months. In 3 (5.8%) patients HK-MoCA score deteriorated from within the normal range at baseline to below normal cutoff at 6 months. The change in cognitive impairment grouping was significant (p < 0.001, Chi-square test). In all, 23 (44.2%) patients had cognitive impairment at 6 months. This cognitively impaired group was older (mean age, 75 ± 12 vs. 70 ± 6 years) and had a lower IADL score (mean, 18 ± 11 vs. 25 ± 4) than the normal group (p < 0.05). They did not differ in HMD score (both baseline and at 6 months), education level, or number of vascular risk factors.
Course of Depression
At 6-month follow-up, the severity of depression as assessed by the HMD was considerably reduced (from 12.3 ± 6.1 to 5.6 ± 4.1, p < 0.001). Applying a HMD score of ≤ 7 as criterion for remission of depression,20 32 (61.5%) patients were considered to be in remission and 20 (38.5%) were not. There was no difference in their age, education level, number of vascular risk factors, IADL score, or cognitive impairment at admission and that at 6 months. Nonetheless mean baseline HMD score (15.55 ± 6.5) and HMD score at 6 months (10.1 ± 2.4) were significantly higher in those not in remission (vs. 10.28 ± 5.0 at baseline and 2.81 ± 1.9 at 6 months in remitted patients). The remission rate was 50% in patients without cognitive impairment and 71.4% in those with cognitive impairment. The difference was not statistically significant.
Discussion
Cognitive impairment constituted a stable feature in a considerable number of elderly patients with depression. Those with greater baseline functional impairment had more persistent cognitive impairment at 6 months. It is possible that cognitive impairment requires a longer time to improve than depressive symptoms. About two-thirds of patients achieved remission of depression after 6 months of treatment.
Our study and the findings of Adler et al11 revealed that in most cases treatment of depressed patients who are cognitively impaired results in improvement in depression after 6 months of treatment, but not cognitive impairment. This is in accordance with other studies that have reported no or only minor improvements in cognitive performance following treatment of late-life depression and recovery from affective symptoms.7,8 Cognitive impairment is common in depressed older adults, especially in those with late onset of depression8 but it has no negative impact on recovery from depression.14 Nearly half of the patients in our study had cognitive impairment at 6 months. Elderly depressed patients with mild cognitive impairment may be considered to be at increased risk of dementia.21 Those who were older and who had a lower functional level at baseline had greater cognitive impairment after 6 months. This supports our hypothesis that functional activity at baseline is a marker for persistent cognitive impairment.12 Executive processes are fundamental to the daily functioning of depressed older adults. Dysfunction might lead to a lack of compensatory strategies that could improve outcomes in late-life depression and thus lead to increased dependency. Executive dysfunction has been strongly linked to IADL impairment in depressed elderly patients.22 Associations between components of executive dysfunction and functional disability have been identified and are independent of depression.23
In this study, the mean HMD score reduced significantly after 6 months of treatment and the rate of remission for depression was 61.5%, similar to that of Adler et al11 and slightly less than that of Tam and Lam.24 This may be due to a difference in definition of remission using the HMD score. The study by Tam and Lam24 used a cutoff score of 10 which was higher than that in our study. The non-remitters had a higher baseline HMD score than the remitters. This concurs with the findings of others where greater severity of depressive episode at baseline predicted poor recovery.24,25 Tam and Lam24 also found that the influence of cognitive deficits on clinical remission became insignificant after controlling for the effects of mood symptoms.
Limitations of this study include the small sample size and the exclusion of patients with late-onset depression who were admitted to a mental hospital. This group of patients would not be followed up by ESPP that targets only community-dwelling elderly with depressive symptoms and a high risk of suicide. Patients who required admission to mental hospital likely had more severe symptoms and a history of suicidal attempt. They thus represented a different group of patients with late-onset depression. Another limitation is the lack of separate analysis for specific components of cognitive functioning.
The results of this study demonstrate the importance of cognitive impairment as a stable feature in a considerable number of elderly individuals with depression. This might suggest an increased risk for the development of dementia. Close monitoring of cognitive functioning is indicated in this group of patients so that early intervention can be offered.
Acknowledgement
We would like to acknowledge the community psychiatric nurses of the Elderly Suicide Prevention Programme of Castle Peak Hospital who have assisted in data collection process of the study. No financial support was received and there were no conflicts of interest.
References
- Gottfries CG. Late life depression. Eur Arch Psychiatry Clin Neurosci 2001;251(Suppl 2):II57-61.
- Chiu PJ, Tetzloff G. EDRF(NO)-mediated modulation of collagen- induced platelet accumulation in rat pulmonary microcirculation. J Biomed Sci 1994;1:43-8.
- Jorm AF. History of depression as a risk factor for dementia: an updated review. Aust N Z J Psychiatry 2001;35:776-81.
- Panza F, Frisardi V, Capurso C, D’Introno A, Colacicco AM, Imbimbo BP, et al. Late-life depression, mild cognitive impairment, and dementia: possible continuum? Am J Geriatr Psychiatry 2010;18:98- 116.
- Caine ED. Pseudodementia. Current concepts and future directions. Arch Gen Psychiatry 1981;38:1359-64.
- Kral VA, Emery OB. Long-term follow-up of depressive pseudodementia of the aged. Can J Psychiatry 1989;34:445-6.
- Butters MA, Becker JT, Nebes RD, Zmuda MD, Mulsant BH, Pollock BG, et al. Changes in cognitive functioning following treatment of late-life depression. Am J Psychiatry 2000;157:1949-54.
- van Reekum R, Simard M, Clarke D, Binns MA, Conn D. Late-life depression as a possible predictor of dementia: cross-sectional and short-term follow-up results. Am J Geriatr Psychiatry 1999;7:151-9.
- The incidence of dementia in Canada. The Canadian Study of Health and Aging Working Group. Neurology 2000;55:66-73.
- Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry 1997;54:915-22.
- Adler G, Chwalek K, Jajcevic A. Six-month course of mild cognitive impairment and affective symptoms in late-life depression. Eur Psychiatry 2004;19:502-5.
- Lee JS, Potter GG, Wagner HR, Welsh-Bohmer KA, Steffens DC. Persistent mild cognitive impairment in geriatric depression. Int Psychogeriatr 2007;19:125-35.
- Devanand DP, Pelton GH, Marston K, Camacho Y, Roose SP, Stern Y, et al. Sertraline treatment of elderly patients with depression and cognitive impairment. Int J Geriatr Psychiatry 2003;18:123-30.
- La Rue A, Spar J, Hill CD. Cognitive impairment in late-life depression: clinical correlates and treatment implications. J Affect Disord 1986;11:179-84.
- Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 1967;6:278-96.
- Wong A, Xiong YY, Kwan PW, Chan AY, Lam WW, Wang K, et al. The validity, reliability and clinical utility of the Hong Kong Montreal Cognitive Assessment (HK-MoCA) in patients with cerebral small vessel disease. Dement Geriatr Cogn Disord 2009;28:81-7.
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969;9:179- 86.
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695-9.
- Yeung PY, Wong LL, Chan CC, Leung JL, Yung CY. A validation study of the Hong Kong version of Montreal Cognitive Assessment (HK-MoCA) in Chinese older adults in Hong Kong. Hong Kong Med J 2014;20:504-10.
- Zimmerman M, Martinez JH, Young D, Chelminski I, Dalrymple K. Severity classification on the Hamilton Depression Rating Scale. J Affect Disord 2013;150:384-8.
- Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, et al. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol 2001;58:397-405.
- Kiosses DN, Klimstra S, Murphy C, Alexopoulos GS. Executive dysfunction and disability in elderly patients with major depression. Am J Geriatr Psychiatry 2001;9:269-74.
- Sanders ML, Lyness JM, Eberly S, King DA, Caine ED. Cerebrovascular risk factors, executive dysfunction, and depression in older primary care patients. Am J Geriatr Psychiatry 2006;14:145-52.
- Tam CW, Lam LC. Clinical remission of late-onset depression in elderly Chinese: a short-term outcome study. East Asian Arch Psychiatry 2013;23:126-32.
- Kok RM, Nolen WA, Heeren TJ. Outcome of late-life depression after 3 years of sequential treatment. Acta Psychiatr Scand 2009;119:274- 81.