East Asian Arch Psychiatry 2021;31:55-66 | https://doi.org/10.12809/eaap2102
ORIGINAL ARTICLE

Yiu-Lung Wong, Department of Psychiatry, Queen Mary Hospital
Calvin Pak-Wing Cheng, Department of Psychiatry, Queen Mary Hospital
Corine Sau-Man Wong, Department of Psychiatry, Queen Mary Hospital
Sze-Nga Wong, Department of Psychiatry, Queen Mary Hospital
Hau-Lam Wong, Department of Psychology, the University of Hong Kong
Serene Tse, Department of Psychiatry, Queen Mary Hospital
Gloria Hoi-Yan Wong, Department of Social Work and Social Administration, The University of Hong Kong
Wai-Chi Chan, Department of Psychiatry, Queen Mary Hospital
Address for correspondence: Calvin Pak-Wing Cheng, Department of Psychiatry, 2/F, New Clinical Building, Queen Mary Hospital, Hong Kong.
Email: chengpsy@hku.hk
Abstract
Objective: We aim to provide an up-to-date systematic review and meta-analysis of the effects of cognitive stimulation (CS) on cognition, depressive symptoms, and quality of life in persons with dementia. Factors affecting the treatment effect were examined.
Methods: A literature search was performed on databases of MEDLINE, EMBASE, PsycINFO, CINAHL Plus, and Cochrane Library up to 7 March 2019. Only randomised controlled trials investigating the effects of CS in persons with dementia were included. The outcome measures were cognitive function, depressive symptoms, and quality of life.
Results: 20 randomised controlled trials with a total of 1251 participants (intervention group: 674; control group: 577) were included for meta-analysis. Most participants had mild to moderate dementia. CS had a significant positive small-to-moderate effect on cognition (Hedges’s g = 0.313, p < 0.001). Heterogeneity of CS was low to moderate (Q=30.5854, df=19, p < 0.05, I2 = 37.877%). Inconclusive results were found for depressive symptoms and quality of life.
Conclusion: CS has a significant positive effect on cognitive function, but its effect on depressive symptoms and quality of life was inconclusive. Future studies with more robust methodology establishing evidence of its efficacy are required.
Key words: Cognition; Dementia; Meta-analysis; Systematic review
Introduction
Dementia is a neurocognitive disorder and a major public health issue1 and causes difficulty in independent daily functioning.2 Dementia has a high burden on morbidity and mortality. Both pharmacological and non-pharmacological treatments are the mainstay of treatment, but pharmacological treatment has potential adverse effects. There is a growing need for non-pharmacological interventions in dementia.3
Cognitive stimulation (CS) aims at stimulating cognitive and social functions globally by means of activities and discussions.4 CS emphasises information processing rather than acquiring knowledge and facts.5 CS can be operationally performed in groups or individually with an integrative approach involving presentation of orientation information, structured discussions on various everyday topics and enjoyable activities within a socially engaged context.4,6,7 The mechanism of action for CS is based on the theories of cognitive reserve and neuronal plasticity, which are originated from reality orientation developed in 1966.8
CS stimulates cognitive functions globally, as various cognitive functions are utilised in a unified rather than an isolated manner.4 However, CS is heterogeneous in terms of formats, settings, and duration of treatment, with high content variability.
Two meta-analyses investigated the effects of CS in dementia.7,9 Both supported the efficacy of CS on cognition (with medium effect size) and on quality of life but not on depressive symptoms. However, other studies reported inconsistent results on cognitive function,10-12 which may be due to the heterogeneity of CS in terms of formats, settings, and duration of treatment, as well as methodological quality of studies, sample size, and recruitment. Depressive symptoms and quality of life were not fully representative in the two meta-analyses. Applying stringent eligibility criteria (pre-defined control group, study design) in selection of studies enables more accurate summarisation of findings. Therefore, we aim to provide an up-to-date systematic review and meta-analysis of the effects of CS on cognition, depressive symptoms, and quality of life in persons with dementia. Factors affecting the treatment effect were examined.
Methods
Only randomised controlled trials (RCTs) with both parallel and cross-over designs were considered. For RCTs with cross-over design, only data before cross-over were included, as it was difficult to ascertain whether the washout period from previous intervention was adequate. CS interventions and reality orientation classified under the domain of CS interventions that fit in definition were included.4
Interventions with different formats (group or individual) and settings (inpatient, outpatient, institution, or home) were accepted. Studies with inactive control group (in form of no active treatment, waitlisted for intervention, or treatment as usual) were included to estimate the absolute effect of treatment.13 Studies with combined interventions (CS +/- other forms of cognitive intervention) were excluded.
Study subjects had to fulfil a diagnosis of dementia. Older studies using descriptive terms other than dementia were considered, but the diagnosis must be supported by cognitive assessment (eg, Mini-Mental State Examination). Studies with a mixed group of subjects (such as mild cognitive impairment and dementia) or subjects with comorbidity (such as delirium, substance misuse, or learning disabilities) were excluded.
Cognitive function had to be assessed using Mini- Mental State Examination or Alzheimer’s Disease Assessment Scale-Cognitive Subscale. Depressive symptoms had to be assessed using the Cornell Scale for Depression in Dementia or the Hamilton Depression Rating Scale. Quality of life had to be measured using Quality of life in Alzheimer’s Disease (QoL-AD). Outcomes immediately after the intervention were measured. Data on follow-up assessments were documented. Studies with no report or inadequate information to calculate the effect size of cognitive outcome were excluded.
A literature search was performed on databases of MEDLINE, EMBASE, PsycINFO, CINAHL Plus, and Cochrane Library (Cochrane Central Register of Controlled Trials (CENTRAL) and Cochrane Database of Systematic Reviews) up to 7 March 2019. No limit was applied for language. Ongoing clinical trials were identified by searching ClinicalTrials.gov, EU Clinical Trials Register, and WHO International Clinical Trials Registry Platform up to 7 March 2019. Reference lists of the included studies and review articles of similar topics were searched to identify potentially eligible studies. Keywords used to identify articles related to CS-related clinical trials in dementia were: exp cognitive therapy/ or cognit* stimulat* therapy* or (cognit* adj (therap* or stimulat* or train* or rehabilitat* or intervent* or enhanc* or enrich* or treat* or activit* or program* or method* or exercise*) or (CST or CS intervent*) or (memory adj (rehabilitat* or strateg* or exercise or train* or group* or enhanc*)) or (((non-drug or non-pharmacologic*) adj3 (treat* or intervent* or program* or method* or therap*)) and cognit*) or reality orient*; exp dementia/ or dement* or Alzheimer* or (frontotemporal lobar degeneration or frontolobar degeneration or frontal lobar degeneration) or vascular cognitive impair* or lewy* bod* or ((memory adj (disorder or impair* or dysfunction)) or (cognitive adj (disorder or impair* or dysfunction)) or neurocognitive disorder); random* or controlled trial or clinical trial or RCT.
Eligible studies were identified by two authors independently. Duplicated records were removed. Studies that met the inclusion criteria or could not be excluded based on titles and abstracts were reviewed in full text. Any disagreements were resolved by discussion or by a third author. The study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.14
The two authors independently extracted the data using a semi-structured form for each trial and cross- checked the results for accuracy. Disagreements regarding data extraction were resolved by consensus or by the third author. The corresponding authors of the studies were contacted for clarification or missing information.
The methodological quality of included RCTs was examined by the three authors independently according to the six domains of the Cochrane Collaboration’s tool for assessing the risk of bias:15 (1) sequence generation, (2) allocation concealment, (3) blinding of the participants, (4) blinding of the assessors, (5) method of addressing incomplete outcome data, and (6) selective reporting. The methodological quality was classified as low, high, or uncertain risk of bias in each domain.
The treatment effect was examined using a meta- analysis by pooling data from the RCTs. For the outcome variables, a pooled effect size was calculated using a random effects model and presented as a standardised mean difference with 95% confidence intervals. The random effects model was used because the estimates of the treatment effect vary between trials because of real differences and sampling variability.16 Forest plots were generated with the Comprehensive Meta-Analysis v3 software.17 Significant heterogeneity between studies was indicated as p < 0.1 in the Q statistic and as >50% in the I2 statistic.18
Subgroup analyses were performed to investigate heterogeneity and estimate treatment effects of CS on cognition for clinically relevant subgroups. Programme formats (individual and group), settings (home and non- home), and duration of treatment were assessed. For the latter, the cut-off point was set at 3 months as it was the median duration among the included studies.
Results
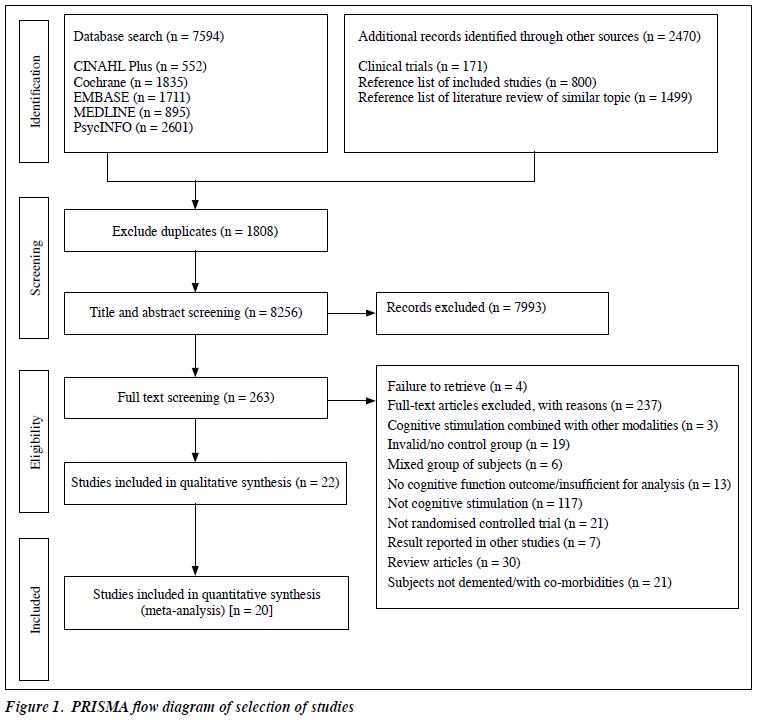
The literature search yielded 7594 results. A detailed PRISMA flow diagram of trails identification and selection is shown in Figure 1. A search of ongoing clinical trials, reference lists of review articles of similar topics, and included studies yielded further 2470 results. After exclusion of duplicates and irrelevant records, 263 articles required full-text review. After excluding the ineligible articles, 22 RCTs were included in the qualitative synthesis.
Cognitive outcome immediately after CS was not available in two RCTs,12,19 despite follow-up through emails, and these two RCTs were excluded. Finally, 20 studies were included in the meta-analysis.11,20-38
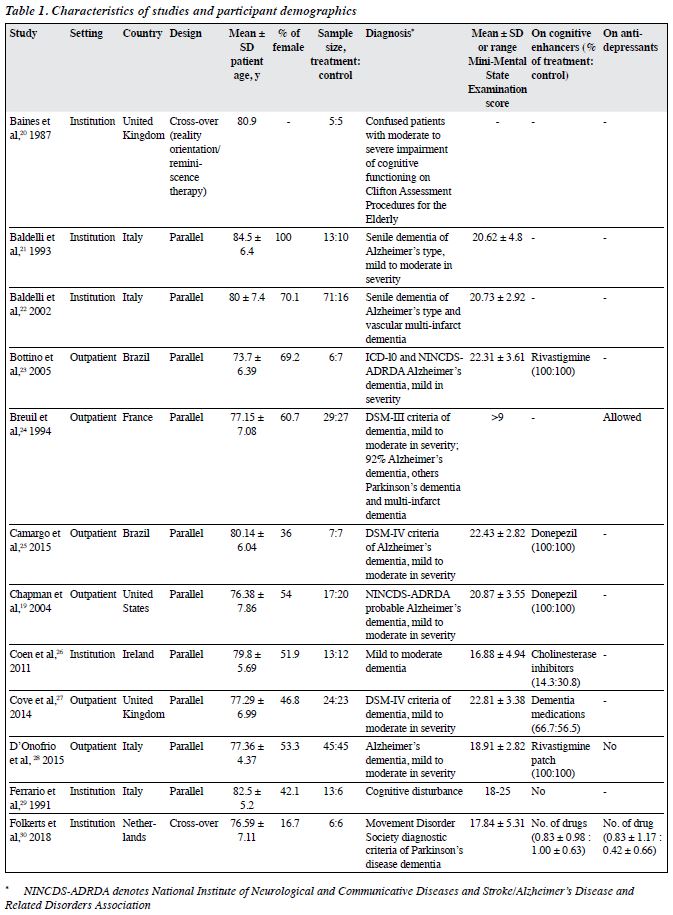
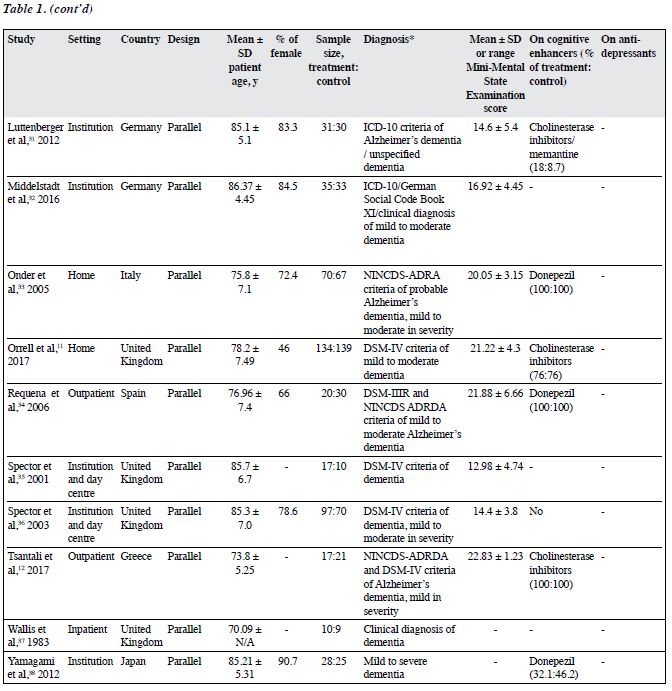
A total of 1343 participants (intervention group: 717, control group: 626) were included (Table 1). Their mean age ranged from 70.09 to 86.37 years. 18 studies reported sex distribution; most comprised predominantly female participants (51.9% to 100%). 15 studies used validated diagnostic guidelines including ICD-10, DSM-III, DSM-III-R, and DSM-IV. The diagnosis of dementia was made based on the Clifton Assessment Procedures for the Elderly and the Mini-Mental State Examination.20,29 14 studies reported the subtypes of dementia, and nearly all of them included those with Alzheimer disease, except for one study that included those with Parkinson dementia only.30 16 studies reported the severity of dementia (mainly mild to moderate dementia). 15 studies reported information on the use of cognitive enhancers: all and none of participants used cognitive enhancers in seven and two studies, respectively. Nearly all studies did not report information on the use of antidepressants.
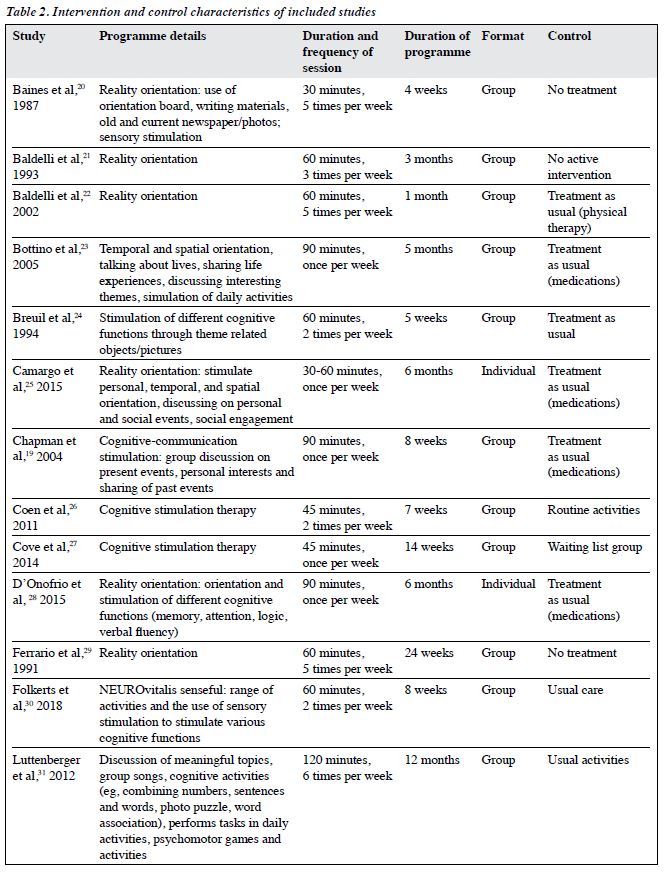
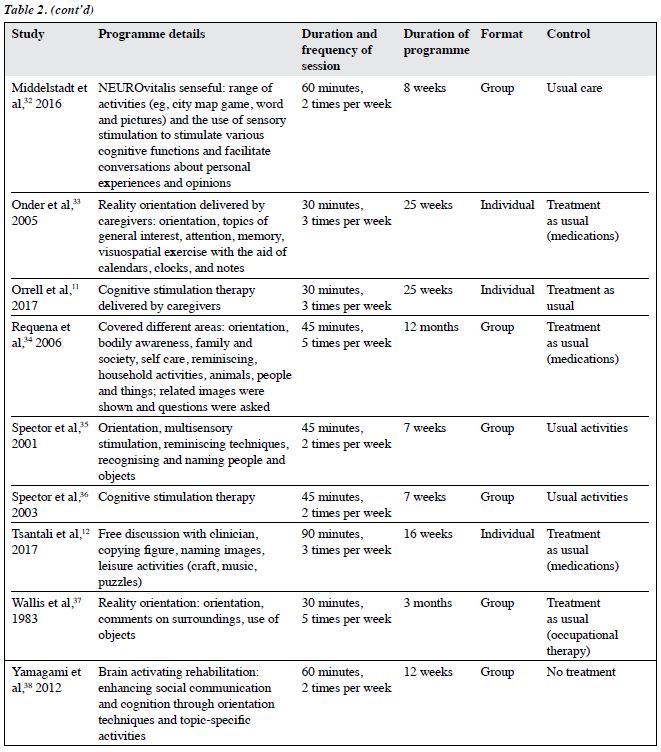
The characteristics of the included RCTs are shown in Table 2. 20 were parallel studies and two were cross- over studies.20,30 CS was commonly carried out in outpatient and institution settings. Only two studies were carried out at home and another two in the hospital. CS therapy was conducted in three studies. Reality orientation was delivered in eight studies. Treatment as usual and no active treatment were the control condition in most studies.
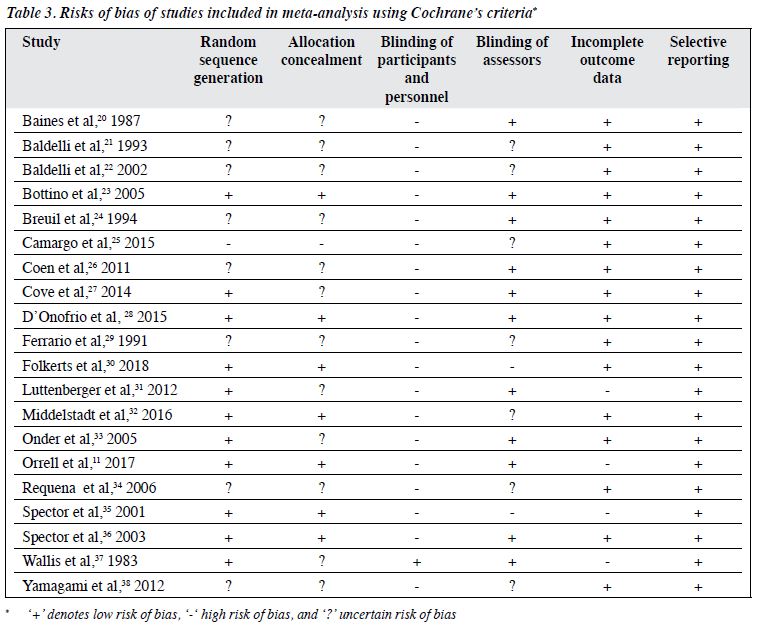
The methodological quality of the included RCTs is summarised in Table 3. In most studies, all types of risk of bias were of low risk, except for blinding of participants and personnel. The risk of bias was uncertain in terms of random sequence generation, adequacy of allocation concealment, and blinding of assessors, because of insufficient information. Four RCTs had a high risk of bias because of having incomplete outcome data. None had a reporting bias. Overall, the methodological quality of included RCTs was moderate, because most types of risk of bias were of low risk, yet uncertain risk was evident for random sequence generation and allocation concealment owing to insufficient information.
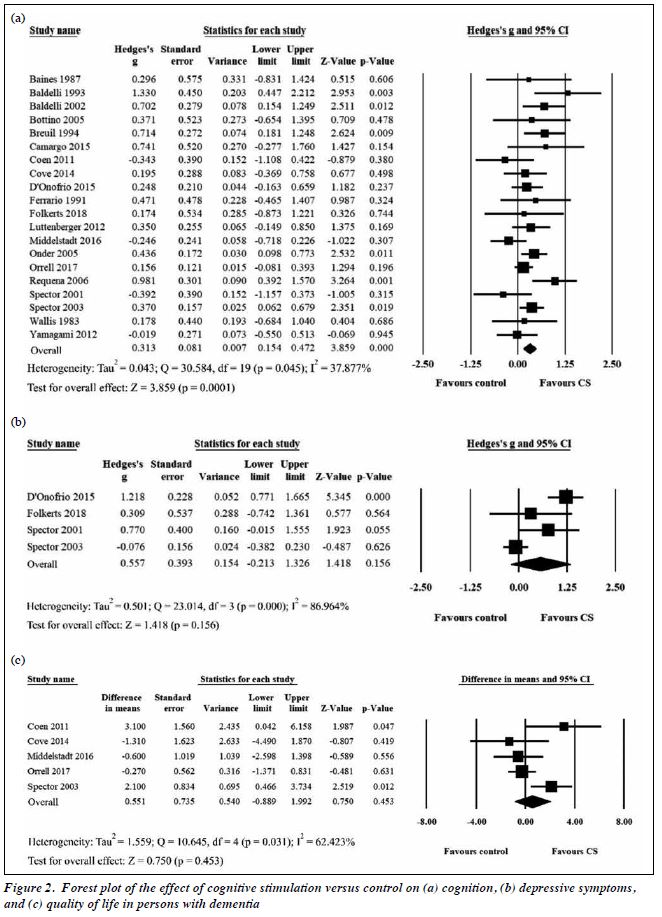
Meta-analysis of 1251 participants (intervention group: 674, control group: 577) in 20 studies indicated that CS had a significant positive small-to-moderate effect on cognition (Hedges’s g = 0.313, 95% CI = 0.154-0.472, p = 0.0001, Figure 2). Heterogeneity of CS was low to moderate (Q = 30.584, df = 19, p = 0.045; I2 = 37.877%). Meta-analysis of 296 participants (intervention group: 165, control group: 131) in four studies indicated that
CS had no significant effect on depressive symptoms (Hedges’s g = 0.557, 95% CI = -0.213 to 1.326, p = 0.156, Figure 2). Meta-analysis of 582 participants (intervention group: 304, control group: 278) in five studies indicated that CS had no significant effect on quality of life (mean difference = 0.551, 95% CI = -0.889 to 1.992, p = 0.453, Figure 2).
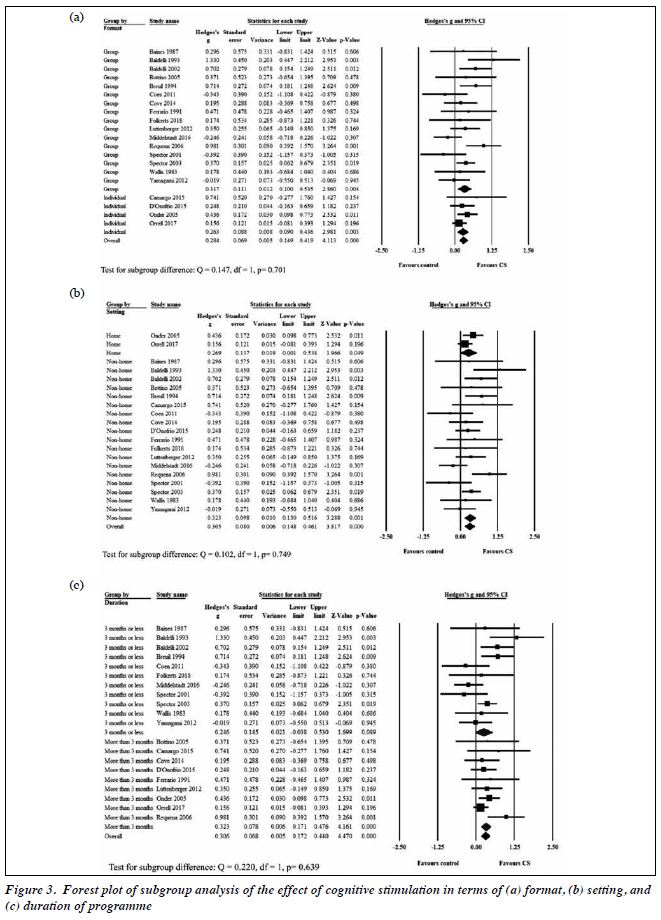
Subgroup analysis of the format of CS indicated no significant difference between individual format (in four studies) and group format (in 16 studies) [Hedges’s g = 0.263 vs 0.317, p = 0.701, Figure 3). Subgroup analysis of the setting of CS indicated no significant difference between home setting and non-home setting (Hedges’s g = 0.269 vs 0.323, p = 0.749, Figure 3). Subgroup analysis of the duration of CS indicated no significant difference between ≤3 months and >3 months (Hedges’s g = 0.246 vs 0.323, p = 0.639, Figure 3).
Egger’s regression tests showed no significant publication bias for the main analyses (p = 0.555), suggesting symmetry in the funnel plot. Sensitivity analysis was performed by omitting studies that used terms other than dementia as diagnosis20,29 and studies that had a high risk of selection bias25 and/or detection bias.30,35 There was no significant change in the primary results of cognition, with similar Hedges’s g.
Discussion
In the present meta-analysis, CS had a significantly positive treatment effect (Hedges’s g = 0.313) on cognition in dementia subjects, compared with inactive controls (including no active treatment, waitlisted for intervention, and treatment as usual). The effect size was smaller than that in other studies (0.417 and 0.449). This is because the present meta-analysis included the largest number of studies and participants and thus had higher power in estimating the effect of CS, particularly a multicentre RCT with nearly 300 participants was included.11 Eight and eleven of the studies were not available in two previous reviews.7,9 We used more stringent eligibility criteria. CS was compared with a consistent pre-defined control group, and only RCTs were included. Definitions for various CS interventions were strictly followed during article selection.4
CS had no significant effect on depressive symptoms. This is because participants might have mild depressive symptoms at baseline (as reflected by the mean score of Cornell Scale for Depression in Dementia) in most included studies30,35,36 and hence the effect of CS, if any, was not apparent. In addition, only four studies that assessed depressive symptoms with appropriate tools were included in meta-analysis. More evidence is needed before any conclusion is reached.
CS had no significant effect on quality of life, although two previous meta-analyses reported a significant improvement in the self-reported quality of life.7,9 This may be due to different types of assessment scales used. The previous meta-analyses used the EuroQol-5-Dimensions and the Quality of Well-being, which are not comprehensive enough to detect the disease-specific changes. We used QoL-AD, which is a valid and reliable scale specifically for assessing the quality of life in persons with (different stages) of dementia.39,40 However, our results on quality of life were not representative because only five studies were included in meta-analysis.
Subgroup analyses suggested no significant subgroup differences in terms of format, setting, and duration of programme. This may be due to the uneven distribution of covariates (the number of trials and study participants in each subgroup). In addition, the investigated characteristics were not exclusive. Other unidentified factors might account for the difference in treatment effect. Participants- related factors could potentially affect the effect size, but meaningful subgroup analyses could not be conducted. For instance, subtypes of dementia could not be investigated, as many studies did not provide related information or analyse separately.
There are some limitations in the present study. The overall quality of the evidence was limited by the methodological quality of included studies. Though the study by Orrell et al11 is the largest clinical trial so far with around 300 participants, the high non-adherence to assigned intervention may cause bias. Heterogeneity could not be explained by the results of subgroup analyses. Nearly all studies were carried out in non-Asian populations; differences in the cultural and social backgrounds could affect the generalisability of the research findings. Meta-analysis on specific cognitive function was not performed. Although there were a few included studies reported assessment in specific cognitive domains such as communication, executive function, and orientation, the high variability of the measurement tools used and scarcity of data make meta- analysis on specific cognitive function of limited value. The impact of various subtypes of dementia on the effectiveness of CS was not addressed. Owing to CS characteristic of involving communication and verbal expression, the effects of CS on different subtypes of dementia may be variable. The underlying pathophysiological mechanisms for respective dementia subtypes may predispose to the differences in the effectiveness of treatment. However, a number of studies did not classify dementia into different subtypes, whereas some studies recruited participants with mixed entities. Therefore, separate meta-analysis for dementia subtypes could not be performed. Stratification of participants by different subtypes of dementia according to consistent diagnostic criteria is suggested for future studies. The sustainability of effect after CS remains uncertain. Given the limited data available together with the arbitrary time points on follow-up assessments, meta-analysis for follow- up assessment was not attempted as it is of limited value with a potential of arriving at an implausible conclusion.
Conclusions
CS has a significant positive effect on cognitive function, but its effect on depressive symptoms and quality of life was inconclusive. The quality of evidence was limited by the methodological quality of included studies and unexplained heterogeneity. Future studies with more robust methodology establishing evidence of its efficacy are required. Stratification of participants by different subtypes of dementia according to consistent diagnostic criteria is recommended. Separate meta-analysis for dementia subtypes is recommended to improve the ability to combine results.
Contributors
All authors designed the study, acquired the data, analysed the data, drafted the manuscript, and critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest. Dr Gloria Wong is the Director of Training of the CST-HK programme.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability
All data generated or analysed during the present study are available from the corresponding author on reasonable request.
References
- World Health Organization. Dementia: a Public Health Priority. Geneva: 2012.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Arlington: 2013. Crossref
- Sopina E, Sørensen J. Decision modelling of non-pharmacological interventions for individuals with dementia: a systematic review of methodologies. Health Econ Rev 2018;8:8. Crossref
- Clare L, Woods RT. Cognitive training and cognitive rehabilitation for people with early-stage Alzheimer’s disease: a review. Neuropsychol Rehabil 2004;14:385-401. Crossref
- Prince M, Bryce R, Ferri C. World Alzheimer Report 2011: the Benefits of Early Diagnosis and Intervention. London: Alzheimer’s Disease International; 2011.
- Choi J, Twamley EW. Cognitive rehabilitation therapies for Alzheimer’s disease: a review of methods to improve treatment engagement and self efficacy. Neuropsychol Rev 2013;23:48-62. Crossref
- Woods B, Aguirre E, Spector AE, Orrell M. Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Sys Rev 2012;2:CD005562. Crossref
- Powell-Proctor L, Miller E. Reality orientation: a critical appraisal. Br J Psychiatry 1982;140:457-63. Crossref
- Kim K, Han JW, So Y, et al. Cognitive stimulation as a therapeutic modality for dementia: a meta-analysis. Psychiatry Investigation 2017;14:626-39. Crossref
- Middelstaedt J, Folkerts AK, Blawath S, Kalbe E. Cognitive stimulation for people with dementia in long-term care facilities: baseline cognitive level predicts cognitive gains, moderated by depression. J Alzheimers Dis 2016;54:253-68. Crossref
- Orrell M, Yates L, Leung P, et al. The impact of individual Cognitive Stimulation Therapy (iCST) on cognition, quality of life, caregiver health, and family relationships in dementia: a randomized controlled trial. PLoS Med 2017;14:e1002269. Crossref
- Tsantali E, Economidis D, Rigopoulou S. Testing the benefits of cognitive training vs. cognitive stimulation in mild Alzheimer’s disease: a randomised controlled trial. Brain Impairment 2017;18:188-96Crossref
- Karlsson P, Bergmark A. Compared with what? An analysis of control- group types in Cochrane and Campbell reviews of psychosocial treatment efficacy with substance use disorders. Addiction 2015;110:420-8. Crossref
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Annals Internal Med 2009;151:264-9. Crossref
- Higgins JP, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; 2019. Crossref
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta- analyses. BMJ 2011;342. Crossref
- Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta- analysis. Res Synthesis Methods 2010;1:97-111. Crossref
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. Crossref
- Chapman SB, Weiner MF, Rackley A, Hynan LS, Zientz J. Effects of cognitive-communication stimulation for Alzheimer’s disease patients treated with donepezil. J Speech Language Hearing Res 2004;47:1149-63Crossref
- Baines S, Saxby P, Ehlert K. Reality orientation and reminiscence therapy: a controlled cross-over study of elderly confused people. Br J Psychiatry 1987;151:222-31. Crossref
- Baldelli MV, Pirani A, Motta M, Abati E, Mariani E, Manzi V. Effects of reality orientation therapy on elderly patients in the community. Arch Gerontol Geriatr 1993;17:211-8. Crossref
- Baldelli MV, Boiardi R, Fabbo A, Pradelli JM, Neri M. The role of reality orientation therapy in restorative care of elderly patients with dementia plus stroke in the subacute nursing home setting. Arch Gerontol Geriatr 2002;Suppl 8:15-22. Crossref
- Bottino CM, Carvalho IA, Alvarez AM, et al. Cognitive rehabilitation combined with drug treatment in Alzheimer’s disease patients: a pilot study. Clin Rehabil 2005;19:861-9. Crossref
- Breuil V, De Rotrou J, Forette F, et al. Cognitive stimulation of patients with dementia: Preliminary results. Int J Geriatr Psychiatry 1994;9:211-7. Crossref
- Camargo CH, Justus FF, Retzlaff G. The effectiveness of reality orientation in the treatment of Alzheimer’s disease. Am J Alzheimers Dis Other Dementias 2015;30:527-32. Crossref
- Coen RF, Flynn B, Rigney E, et al. Efficacy of a cognitive stimulation therapy programme for people with dementia. Irish J Psychol Med 2011;28:145-7. Crossref
- Cove J, Jacobi N, Donovan H, Orrell M, Stott J, Spector A. Effectiveness of weekly cognitive stimulation therapy for people with dementia and the additional impact of enhancing cognitive stimulation therapy with a carer training program. Clin Interventions Aging 2014;9:2143-50. Crossref
- D’Onofrio G, Sancarlo D, Addante F, et al. A pilot randomized controlled trial evaluating an integrated treatment of rivastigmine transdermal patch and cognitive stimulation in patients with Alzheimer’s disease. Int J Geriatr Psychiatry 2015;30:965-75. Crossref
- Ferrario E, Cappa G, Molaschi M, Rocco M, Fabris F. Reality orientation therapy in institutionalized elderly patients: Preliminary results. Arch Gerontol Geriatr 1991;Suppl 2:139-42.
- Folkerts AK, Dorn ME, Roheger M, et al. Cognitive stimulation for individuals with Parkinson’s disease dementia living in long-term care: preliminary data from a randomized crossover pilot study. Parkinsons Dis 2018;8104673. Crossref
- Luttenberger K, Hofner B, Graessel E. Are the effects of a non-drug multimodal activation therapy of dementia sustainable? Follow-up study 10 months after completion of a randomised controlled trial. BMC Neurology 2012;12:151. Crossref
- Middelstaedt J, Folkerts AK, Blawath S, Kalbe E. Cognitive stimulation for people with dementia in long-term care facilities: baseline cognitive level predicts cognitive gains, moderated by depression. J Alzheimers Dis 2016;54:253-68. Crossref
- Onder G, Zanetti O, Giacobini E, et al. Reality orientation therapy combined with cholinesterase inhibitors in Alzheimer’s disease: Randomised controlled trial. Br J Psychiatry 2005;187:450-5. Crossref
- Requena C, Maestu F, Campo P, Fernandez A, Ortiz T. Effects of cholinergic drugs and cognitive training on dementia: 2-year follow- up. Dementia Geriatr Cogn Disord 2006;22:339-45. Crossref
- Spector A, Orrell M, Davies S, Woods B. Can reality orientation be rehabilitated? Development and piloting of an evidence-based programme of cognition-based therapies for people with dementia. Neuropsychol Rehabil 2001;11:377-97. Crossref
- Spector A, Thorgrimsen L, Woods BOB, et al. Efficacy of an evidence- based cognitive stimulation therapy programme for people with dementia: randomised controlled trial. Br J Psychiatry 2003;183:248-54Crossref
- Wallis GG, Baldwin M, Higginbotham P. Reality orientation therapy: a controlled trial. Br J Med Psychol 1983;56:271-7. Crossref
- Yamagami T, Takayama Y, Maki Y, Yamaguchi H. A randomized controlled trial of brain-activating rehabilitation for elderly participants with dementia in residential care homes. Dementia Geriatr Cogn Disord Extra 2012;2:372-80. Crossref
- Bowling A, Rowe G, Adams S, et al. Quality of life in dementia: a systematically conducted narrative review of dementiaspecific measurement scales. Aging Mental Health 2015;19:13-31. Crossref
- Thorgrimsen L, Selwood A, Spector A, et al. Whose quality of life is it anyway? The validity and reliability of the Quality of Life-Alzheimer’s Disease (QoL-AD) scale. Alzheimer Dis Assoc Disord 2003;17:201-8Crossref