East Asian Arch Psychiatry 2023;33:71-6 | https://doi.org/10.12809/eaap2309
ORIGINAL ARTICLE
Abstract
Objective: Anhedonia, commonly defined as a reduced ability to feel pleasure, is a core clinical symptom of late-life depression (LLD). Deficits in reward processing are hypothesised to be associated with anhedonia. We examined differences in reward sensitivity between patients with LLD and healthy controls and explored the associations between LLD-related symptomatology, global cognition, and the reward system.
Methods
The reward responsiveness of 63 patients with LLD and 58 healthy controls aged ≥60 years was assessed using the probabilistic reward learning task with an asymmetric reward schedule.
Results: Compared with healthy controls, patients with LLD displayed lower response bias and reward learning. Global cognition of all participants was positively correlated with response bias. In patients with LLD, anhedonia severity explained impaired reward learning.
Conclusion: A deficit in reward processing is implicated in patients with LLD. Our findings suggest that executive dysfunction and anhedonia contribute to lower sensitivity to reward learning in patients with LLD.
Sze Ting Joanna Ngan, Department of Psychiatry, The University of Hong Kong, Hong Kong SAR, China
Wai Chi Chan, Department of Psychiatry, Queen Mary Hospital, Hong Kong SAR, China
Shu Ting Wong, Department of Psychiatry, The University of Hong Kong, Hong Kong SAR, China
Corine Sau Man Wong, Division of Community Medicine and Public Health Practice, School of Public Health, The University of Hong Kong, Hong Kong SAR, China
Calvin Pak Wing Cheng, Department of Psychiatry, The University of Hong Kong, Hong Kong SAR, China
Address for correspondence: Dr Calvin Pak Wing Cheng, Department of Psychiatry, The University of Hong Kong, Hong Kong SAR, China. Email: chengpsy@hku.hk
Submitted: 28 February 2023; Accepted: 7 June 2023
Introduction
Late-life depression (LLD) is defined as a depressive episode occurring at or after the age of 60 or 65 years; the precise age varies in different studies.1 In both the ICD-10 and DSM-5, clinical symptoms of depression include persistent low mood and anhedonia.2 Anhedonia is defined as a reduced ability to feel pleasure and is commonly experienced by patients with LLD.3,4 It is a significant prognostic factor for treatment outcomes and overall functioning.5,6 Anhedonia is characterised as a trait marker rather than a state marker, being relatively persistent in those being treated with antidepressants.7 The precise mechanism of anhedonia is not fully understood, but it is believed to be closely related to a dysfunctional reward system.8,9
Reward processing is a complex system that involves many components. Problems related to the reward system that are seen in depression include reduced reward learning and maladaptive responses to reward.8,10 In the integrative model of ageing and dopaminergic dysfunction, increased levels of inflammatory cytokines, including tumour necrosis factor, in the blood and cerebrospinal fluid of patients with LLD are associated with anhedonia severity and reduced motivation.11
Reduction in reward learning is a reduced ability to integrate past reinforcement information, which results in conservative reward-related decision making12; it has been shown to be positively correlated with the severity of anhedonia.10 Dysfunctional reward systems may also lead to other LLD symptoms such as negative thoughts and suicidal ideas or attempts.13,14 Reward learning and sensitivity have been examined using the probabilistic reward learning task (PRT) delivered via the E-Prime in both adults with depression and healthy undergraduate students; the PRT is shown to have adequate test-retest reliability, convergent validity, and predictive validity.10,12,15 Patients with high levels of anhedonia or depressive symptoms have reduced reward learning, compared with controls. However, no study has examined reward learning using PRT in patients with LLD.
Two studies investigated reward-related decision making in LLD using the Iowa Gambling Task.16,17 Patients with LLD, particularly those with apathy, performed more conservatively than controls, probably owing to their reduced sensitivity to reward. There is evidence to suggest that impairment in the reward system in patients with LLD is associated with some clinical symptoms.18-20 However, the associations between sensitivity to reward and clinical symptoms of LLD remain largely unknown. Better understanding of the deficits in the reward system of patients with LLD and their associations with clinical symptoms may help to explain the role of the reward system in the pathophysiology of LLD and provide new directions to treatment and management of LLD.
The present study aimed to examine differences in reward sensitivity between patients with LLD and healthy controls. We hypothesised that patients would have reduced response bias in the PRT due to insensitivity to reward. In addition, the associations between LLD-related symptomatology, global cognition, and reward system were determined. We hypothesised that reduced reward learning would be associated with levels of anhedonia in patients with LLD. The findings on psychopathological mechanisms may help to provide a new direction in the treatment and management of LLD.
Methods
A total of 121 Chinese older adults aged ≥60 years (63 patients with LLD and 58 healthy controls) were recruited. Patients with a primary diagnosis of major depressive disorder (MDD) who had not had a change in their antidepressant dosage for at least 2 weeks and were screened by psychiatrists from the outpatient psychiatric clinics of the Queen Mary hospital in Hong Kong using the DSM-5 Structured Clinical Interview were included. Those with a diagnosis other than MDD such as major neurocognitive disorder of any type including Alzheimer disease, those with a score on the Hong Kong Chinese version of the Montreal Cognitive Assessment (HK- MoCA)21 below the second percentile relative to the patient age and education level, or those with comorbid alcohol or substance dependence or any concomitant unstable major medical or neurological conditions were excluded. Healthy controls who had no personal or family history of mental illness were recruited from the community. Face- to-face interviews were conducted to screen for normal or corrected-to-normal vision.
Demographic data including age, sex, and years of education were collected. Depressive symptoms were assessed using the Hamilton Depression Rating Scale (HAM-D-17).22 Anhedonia was assessed using the Chinese version of the Snaith-Hamilton Pleasure Scale (SHAPS) [Cronbach’s α = 0.90].23,24 Global cognition was measured using the HK-MoCA (Cronbach’s α = 0.77).21 Working memory and attention were measured using the forward and backward Digit Span. Executive function was assessed using the Trail Making Test (TMT) and Verbal Fluency Test (VFT).
The PRT was run using E-Prime 1.2 (Psychology Software Tools, Pittsburgh, PA, USA) to quantify reward responsiveness. The PRT consists of three blocks of 100 trials and lasts for approximately 15 minutes. Each trial begins with a mouthless face for 500 ms, followed by either a short (11.5 mm) or long (13 mm) mouth appearing for 100 ms. Participants were asked to press the key ‘E’ when they saw a short mouth and ‘I’ for a long mouth. Correct responses received a monetary reward 40% of the time. Participants were informed that correct responses would not always be rewarded and were asked to win as much money as possible. However, participants were not informed that an asymmetrical reinforcement schedule was used to assess response bias; a ‘rich stimulus’ having a 3-fold greater chance of being rewarded for one type of mouth and a ‘lean stimulus’ for another type of mouth were randomised in each block. Response bias was expected in healthy controls with greater reward responsiveness to favour the ‘rich stimulus’.
Outlier responses were excluded, including reaction times <150 ms and >1500 ms, trials of each participant with ±3 standard deviations of mean, and those with more than 30 trials of outliers. Task performance was quantified according to discrimination performance. Response bias was used to assess performance, using participants’ preference for the rich stimulus (see formula below).10 A high response bias was evident when participants had higher rates of correct identification for the rich stimuli and higher miss rates for lean stimuli. Reward learning was assessed by the difference in response bias between blocks (ie, block 3 – block 2).
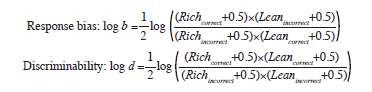
Demographic, clinical, cognitive, and discriminability data were compared between patient and control groups using independent samples t tests for parametric variables and Mann-Whitney U tests for nonparametric variables. To explore group differences across blocks, response bias and reward learning were analysed using the generalised linear model, controlled for age, sex, and education years. P values were adjusted using the Benjamini-Hochberg false discovery rate (FDR) correction procedure for multiple testing (FDR = 0.1).
Given the nonparametric nature of the variables, Spearman correlation analyses were performed to examine the associations between anhedonia and response learning and between cognitive function and response bias. Linear regression was used to analyse response bias and reward learning, with cognitive measures and symptomatology measures that reflects a significant group difference as independent variables. The association between anhedonia and PRT performance in response bias and reward learning was explored. All tests were two-tailed with the significance level set at p < 0.05. Analyses were performed using SPSS (Windows version 27.0; IBM Corp, Armonk [NY], US).
Results
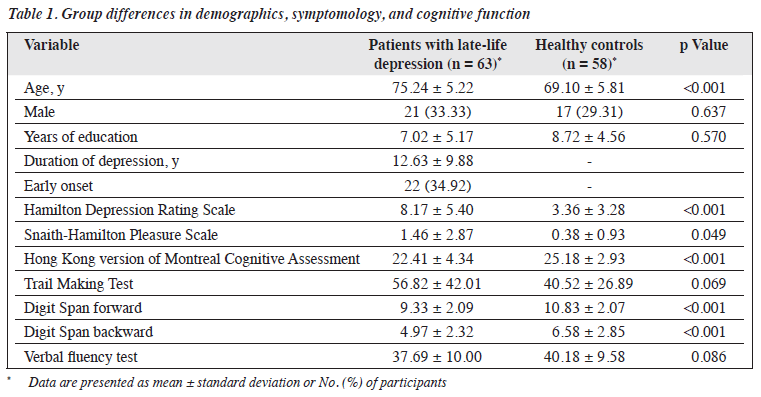
The patient and control groups were comparable in terms of sex or education years, but the patient group was significantly older than the control group (Table 1). The control group performed significantly better on the HK-MoCA and Digit Span forward and backward tests. The patient group had higher HAM-D score for depression and SHAPS score for anhedonia.
The two groups were comparable in discriminability (all F < 0.56, all p > 0.05). Mixed analysis of variance revealed no significant group difference in response bias between blocks (F2,234 = 1.226, p = 0.295). However, there was a trend of greater response bias in healthy controls than patients with LLD (Figure 1).
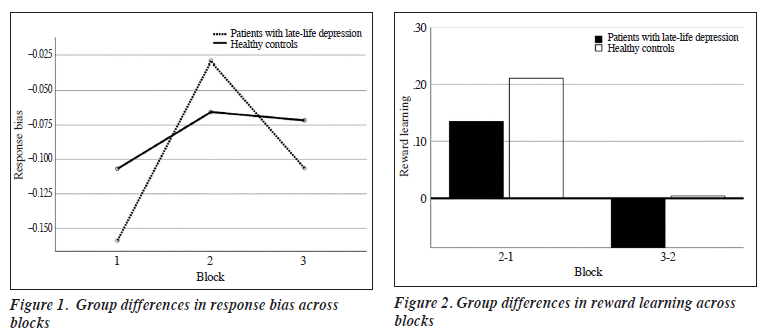
No significant group difference was found in response learning between blocks (F2,234 = 2.516, p = 0.115, Figure 2). Reward learning was slightly better in patients, but this trend was reversed in latter trials.
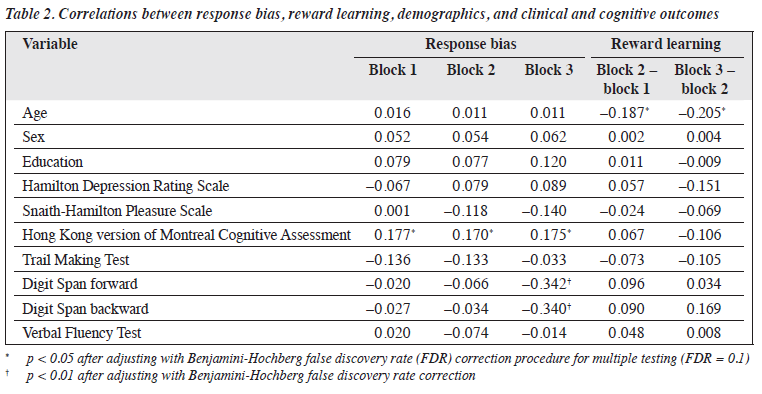
Spearman rank correlation analysis revealed that age was negatively correlated with reward learning but not sex or education (Table 2). Only SHAPS scores were correlated with reward learning in the later blocks (block 3 − block 2). No association was found between HAM-D scores and response bias or reward learning. HK-MoCA scores were correlated with response bias in all three blocks (p < 0.005) but not other cognitive measures. A linear regression analysis was conducted to examine the association between Digit Span forward and backward scores and response bias. Both Digit Span forward (β = −0.002, t5 = −0.341, p < 0.001) and backward (β = −0.002, t5 = −0.343, p < 0.001) scores were predictors of response bias in block 3, after controlling for age, education years, sex, and HK-MoCA scores. The regression equation for Digit Span forward scores was significant (F5,115 = 3.758, p = 0.003) and accounted for 14% of the variance in response bias. Similarly, the regression equation for the Digit Span backward scores was significant (F5,115 = 3.813, p = 0.004) and accounted for 14% of the variance in response bias.
No significant association was found between SHAPS scores and PRT performance. However, when examining patients with LLD and controlling for age, sex, education years, and HAM-D score, SHAPS scores explained reward learning in latter trials (block 3 − block 2) [β = −2.55, p = 0.022]. The regression equation for SHAPS scores was significant (F5,57 = 3.040, p = 0.017) and accounted for the variance in reward learning (R2 = 0.221). No significant association was found between SHAPS scores in heathy controls and reward learning (β = −0.106, p = 0.285).
Discussion
The current study is the first to explore reward learning using PRT in patients with LLD. It examined differences in response bias and reward learning between patients with LLD and healthy controls. There were trends of reduced response bias and reward learning in patients with LLD. Global cognition was correlated with response bias in both groups. Similarly, measures for working memory and attention explained response bias in both groups. In patients with LLD, anhedonia severity explained impaired reward learning.
In contrast to previous studies involving adolescents and adults,5,12 our study with older adults aged ≥60 years had an overall lower level of response bias and reward learning. This may be explained by age-related cognitive deficits in reward processing.25 Indeed, our study found that age was negatively correlated with reward learning, whereas global cognition scores were positively correlated with response bias. As reinforcement learning is correlated with global cognition, those with impaired reinforcement learning, which is prevalent in older adults, would require more time in learning to detect response bias.26 This may explain why our participants, unlike working-age participants in previous studies, required additional time to learn the asymmetric reward schedule.
The PRT performance between patients with LLD and healthy controls did not differ significantly. One possible reason may be the attentional deficits and cognitive difficulties experienced by older adults. It is speculated that a deficit in attentional function could explain the reduced reward learning over time (across blocks). The reduction in both response bias and reward learning was especially prominent in patients with LLD. This may be partially due to impairments in executive function and cognitive control.27 Forward and backward Digit Span scores, which assessed working memory and attention, respectively, attributed to response bias in the last block. This confirmed the role of executive function in explaining the impaired response bias in patients with LLD.
Another explanation for the insignificant group difference may be the limited range in severity of depression among patients. Most patients had only mild depression, with only four having a high anhedonia score (SHAPS score >7). This varies from a study in which the depressed group had a much higher mean SHAPS score, with 55.6% of patients having a high anhedonia score (SHAPS >7).10 As a result, the limited range of depression severity may have contributed to the insignificant group difference in task performance.
Nonetheless, a trend of reduced response bias and reward learning was revealed in the last blocks, consistent with a previous study of patients with LLD.10 The generally lower response bias in patients with LLD may reflect a lower sensitivity to reward.19 The reduced reward learning in the last block also suggests that impaired reinforcement learning, as seen in adults with MMD, also extends to patients with LLD.26
Consistent with previous studies, the level of anhedonia in patients with LLD predicted the decreased reward learning in the last block, even after adjusting for depressive symptoms. The underlying mechanism of depression was further elucidated that depression was indicated by the impaired ability to modulate behaviour according to past reinforcement history. This includes the processes related to reward sensitivity and reward learning. As rewards are positive reinforcers and promote the enactment of behaviours, blunted responsiveness to reinforcers — as experienced by patients with anhedonia — may reduce sensitivity to rewards, making such patients less prone to engaging in goal-directed behaviours.28 As a result, continuous disengagement may reinforce the maintenance of depression or worsen the patient’s prognosis.29,30 Our findings confirmed the underlying reward mechanism of depression by identifying how anhedonia may interfere with reward learning.
There were some limitations to this study. Owing to the cognitive demand of the PRT and the use of only three testing blocks, the delayed learning seen in our older adults may have contributed to the low level of response bias. This may reduce the sensitivity of the test and its ability to detect group differences in response bias. In addition, the small sample size may be problematic for pattern recognition among the participants.31 Further analysis of differences in age at onset in patients with LLD was not performed. Further research is needed to examine differences between patients with early-onset and late-onset depression and the associations between the reward system and executive functioning in these groups. Future studies using the PRT could include additional blocks of reinforcement learning to allow for more learning and response time and thus to confirm the difference in response bias between patients with LLD and healthy controls.
Conclusion
There was a trend towards impaired reward learning in patients with LLD, compared with healthy controls. A degree of executive dysfunction and anhedonia among the participants may have decreased the sensitivity of the PRT and the ability to detect differences in reward and learning in patients with LLD. Given the negative impact of anhedonia on treatment resistance and general wellbeing, further studies with more trials to account for the cognitive deficits seen in older adults are needed to investigate the effect of anhedonia.
Contributors
STJN drafted and prepared the manuscript. STJN and STW contributed to the data analysis. STJN, CPWC, and CSMW contributed to the interpretation of data. CPWC and WCC contributed to the conception and design of the study. All authors reviewed the results and approved the final version of the manuscript.
Conflicts of Interest
As an editor of the journal, CSM Wong was not involved in the peer review process. Other authors have disclosed no conflicts of interest.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability
All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval
The study was approved by Institutional Review Board of The University of Hong Kong / Hospital Authority Hong Kong West Cluster (reference: UW 18-137). The patients were treated in accordance with the tenets of the Declaration of Helsinki. The patients provided written informed consent for all treatments and procedures.
References
- Hall CA, Reynolds-Iii CF. Late-life depression in the primary care setting: challenges, collaborative care, and prevention. Maturitas 2014;79:147-52. Crossref
- Sibitz I, Berger P, Freidl M, et al. ICD-10 or DSM-IV? Anhedonia, fatigue and depressed mood as screening symptoms for diagnosing a current depressive episode in physically ill patients in general hospital. J Affect Disord 2010;126:245-51. Crossref
- Ho N, Sommers M. Anhedonia: a concept analysis. Arch Psychiatr Nurs 2013;27:121-9. Crossref
- Lam L, Tam C. Cognitive function, functional performance and severity of depression in Chinese older persons with late-onset depression. East Asian Arch Psychiatry 2012;22:12-7.
- Vrieze E, Demyttenaere K, Bruffaerts R, et al. Dimensions in major depressive disorder and their relevance for treatment outcome. J Affect Disord 2014;155:35-41. Crossref
- Llorca P, Gourion D. Management of anhedonia and depressive symptoms in depressed outpatients: benefit for functioning. Eur Psychiatry 2015;30(Suppl 1):364. Crossref
- Di Nicola M, De Risio L, Battaglia C, Camardese G, Tedeschi D, Mazza M, et al. Reduced hedonic capacity in euthymic bipolar subjects: a trait-like feature? J Affect Disord 2013;147:446-50. Crossref
- Nassima B, Souheyla B. Reward and punishment processing in depression. Int J Humanit Soc Sci Educ 2014;1:63-8.
- Höflich A, Michenthaler P, Kasper S, Lanzenberger R. Circuit mechanisms of reward, anhedonia, and depression. Int J Neuropsychopharmacol 2019;22:105-18. Crossref
- Vrieze E, Pizzagalli DA, Demyttenaere K, et al. Reduced reward learning predicts outcome in major depressive disorder. Biol Psychiatry 2013;73:639-45. Crossref
- Rengasamy M, Marsland A, McClain L, et al. Longitudinal relationships of cytokines, depression and anhedonia in depressed adolescents. Brain Behav Immun 2021;91:74-80. Crossref
- Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res 2008;43:76-87. Crossref
- Victoria LW, Gunning FM, Bress JN, Jackson D, Alexopoulos GS. Reward learning impairment and avoidance and rumination responses at the end of Engage therapy of late-life depression. Int J Geriatr Psychiatry 2018;33:948-55. Crossref
- Dombrovski AY, Szanto K, Clark L, Reynolds CF, Siegle GJ. Reward signals, attempted suicide, and impulsivity in late-life depression. JAMA Psychiatry 2013;70:1. Crossref
- Pizzagalli DA, Jahn AL, O’Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry 2005;57:319-27. Crossref
- Alexopoulos GS, Manning K, Kanellopoulos D, et al. Cognitive control, reward-related decision making and outcomes of late-life depression treated with an antidepressant. Psychol Med 2015;45:3111-20.Crossref
- McGovern AR, Alexopoulos GS, Yuen GS, Morimoto SS, Gunning- Dixon FM. Reward-related decision making in older adults: relationship to clinical presentation of depression. Int J Geriatr Psychiatry 2014;29:1125-31. Crossref
- Taylor WD, Zald DH, Felger JC, et al. Influences of dopaminergic system dysfunction on late-life depression. Mol Psychiatry 2022;27:180-91. Crossref
- Alexopoulos GS. Mechanisms and treatment of late-life depression. Transl Psychiatry 2019;9:188. Crossref
- Solomonov N, Victoria LW, Dunlop K, et al. Resting state functional connectivity and outcomes of psychotherapies for late-life depression. Am J Geriatr Psychiatry 2020;28:859-68. Crossref
- Wong A, Yiu S, Nasreddine Z, et al. Validity and reliability of two alternate versions of the Montreal Cognitive Assessment (Hong Kong version) for screening of mild neurocognitive disorder. PLoS One 2018;13:e0196344. Crossref
- Zheng YP, Zhao JP, Phillips M, et al. Validity and reliability of the Chinese Hamilton Depression Rating Scale. Br J Psychiatry 1988;152:660-4. Crossref
- Zhang P, Zhang N, Fang S, et al. Factor structure and measurement invariance of the Chinese version of the Snaith-Hamilton Pleasure Scale (SHAPS) in non-clinical and clinical populations. J Affect Disord 2021;281:759-66. Crossref
- Liu WH, Wang LZ, Zhu YH, Li MH, Chan RC. Clinical utility of the Snaith-Hamilton-Pleasure scale in the Chinese settings. BMC Psychiatry 2012;12:184. Crossref
- Schott BH, Niehaus L, Wittmann BC, et al. Ageing and early-stage Parkinson’s disease affect separable neural mechanisms of mesolimbic reward processing. Brain 2007;130:2412-24. Crossref
- Cutler J, Wittmann MK, Abdurahman A, et al. Ageing is associated with disrupted reinforcement learning whilst learning to help others is preserved. Nat Commun 2021;12:4440. Crossref
- Rajtar-Zembaty A, Sałakowski A, Rajtar-Zembaty J, Starowicz- Filip A. Executive dysfunction in late-life depression. Psychiatr Pol 2017;51:705-18. Crossref
- Recorla RA, Wagner AR. A Theory of Pavlovian Conditioning: Variations in the Effectiveness of Reinforcement and Nonreinforcement. In: Black AH, Prokasy WF, editors. Classical Conditioning II: Current Research and Theory. New York: Appleton- Century-Crofts; 64-99.
- Kasch KL, Rottenberg J, Arnow BA, Gotlib IH. Behavioral activation and inhibition systems and the severity and course of depression. J Abnorm Psychol 2002;111:589-97. Crossref
- Miller CH, Davis EG, King LS, Sacchet MD, Grill-Spector K, Gotlib IH. The structure of depressive symptoms and characteristics and their relation to overall severity in major depressive disorder. Psychiatry Res 2020;294:113399. Crossref
- Kanal L, Chandrasekaran B. On dimensionality and sample size in statistical pattern classification. Pattern Recognit 1971;3:225-34. Crossref