East Asian Arch Psychiatry 2024;34:122-7 | https://doi.org/10.12809/eaap2445
ORIGINAL ARTICLE
Abstract
Background: Endophenotypes aid in studying the complex genetic basis of bipolar disorder. We aimed to compare first-degree relatives of patients with bipolar I disorder in a hospital in India with unrelated healthy controls in terms of neurocognition and affective temperament.
Methods: This cross-sectional study was conducted between August and November 2012 at a tertiary hospital in India. First-degree relatives (parents, siblings, and children) of patients with bipolar I disorder were included; they were aged 18 to 50 years and had education level of at least eighth grade. Additionally, matched healthy controls were recruited from the general population. Sociodemographic data were collected using a semi-structured proforma. Participants were assessed for verbal and visual working memory, executive function (including cognitive flexibility, response inhibition, as well as concept formation, abstract reasoning, and set-shifting abilities), and affective temperament by a single investigator.
Results: Of the 52 first-degree relatives of patients diagnosed with bipolar I disorder, 30 were included in the analysis. Additionally, 30 matched healthy controls from the general population were included for comparison. Compared with healthy controls, first-degree relatives performed significantly poorer in all tests and had significantly higher scores for cyclothymic, hyperthymic, and anxious temperaments.
Conclusion: Impairments in working memory, executive function, and certain affective temperaments are potential endophenotypes for bipolar I disorder. Working memory and executive function are most important cognitive domains for social, occupational, and interpersonal functioning. These potential markers could be used to trace susceptible genes for bipolar disorder and thus enhance our understanding of the complex genetics of mood disorders.
Thenmozhi Lakshmanamoorthy, Department of Psychiatry, All India Institute of Medical Sciences-Madurai, Ramanathapuram, Tamil Nadu, India
Bharathi Rajendran, Department of Psychiatry, Government Medical College and Hospital, Ramanathapuram, Tamil Nadu, India
Arumuganathan Shanmugavinayagam, Department of Psychiatry, All India Institute of Medical Sciences-Madurai, Ramanathapuram, Tamil Nadu, India
Praveena Daya Appadurai, Department of Community and Family Medicine, All India Institute of Medical Sciences-Madurai, Ramanthapuram, Tamil Nadu, India
Ayyakutti Muni Raja, Department of Ophthalmology, All India Institute of Medical Sciences-Madurai, Ramanthapuram, Tamil Nadu, India
Rajeswari Kathiah, Department of Pathology, All India Institute of Medical Sciences-Madurai, Ramanthapuram, Tamil Nadu, India
Address for correspondence: Dr Thenmozhi Lakshmanamoorthy, Department of Psychiatry, All India Institute of Medical Sciences-Madurai, Ramanathapuram, Tamil Nadu, India. Email: dr.then16@gmail.com
Submitted: 31 August 2024; Accepted: 30 October 2024
Psychiatric disorders arise from a multitude of biological and environmental factors. Bipolar disorder is characterised by mood swings from manic highs to depressive lows; functional impairment occurs even in patients who are in remission. It is important to determine how genetic factors affect psychiatric disorders (genotype to phenotype), but psychiatry has had limited success in identifying the responsible gene or gene areas. Thus, the term endophenotype is used to bridge the gap between genotype and phenotype.
An endophenotype is defined as internal phenotype discoverable by microscopic examination or biochemical test.1 Synonymous terms include intermediate phenotype, biological marker, subclinical trait, and vulnerability marker. Endophenotypes may help to identify the aberrant genes in the polygenic systems that are vulnerable to disorders. Endophenotypes are associated with illness in the population; they are heritable, state-independent, co-segregated with illness within families and have higher prevalence in non-affected family members than in the general population.1 Endophenotype studies can facilitate the identification of genes of potential risk and link them to neurobiological and neurophysiological traits that underlie a psychiatric disorder. This shifts the focus from gene discovery to functional characterisation.2 Methods of analysis include biochemical, neurophysiological, endocrinological, neuroanatomical, cognitive, and neuropsychological techniques. Candidate endophenotypes of schizophrenia, mood disorders, Alzheimer disease, attention deficit hyperactivity disorder, and personality disorders have been studied.
Genetics have strong influences on attention, executive functioning, speed of processing, working memory, and declarative memory. The heritability estimate of attention is reported to be 41% to 49%,3 whereas for cognitive flexibility in the Wisconsin Card Sorting Test, 37% to 46%,4 and for working memory, 43% to 49%.5
Neurocognitive studies of patients with bipolar disorder demonstrate impairment in the cognitive domains of attention, verbal memory, and executive function.6 These impairments are present during both the illness and euthymic phases.7,8 Cognitive studies in unaffected relatives of patients with bipolar disorder have identified deficits in various cognitive domains, most commonly in working memory and executive function.9,10
Temperament is defined as a person’s predisposition towards certain patterns of reactivity, mood, and sensitivity that are stable over time and heritable.11,12 Bipolar disorder is associated with affective temperament, particularly cyclothymic, hyperthymic, and depressed temperaments.13 These associations remain during the euthymic phase of bipolar disorder.14 This endophenotype (ie, cyclothymic and hyperthymic temperament) is also found in unaffected family members of patients with bipolar disorder.13,15
Most studies of neurocognition and affective temperament as endophenotypes in bipolar disorder have been in Western populations. We aimed to compare first- degree relatives of patients with bipolar I disorder in a hospital in India with unrelated healthy controls in terms of neurocognition and affective temperament.
Methods
This cross-sectional study was conducted between August and November 2012 at a tertiary hospital in Tamil Nadu, India. Consecutive patients were screened for diagnosis of bipolar I disorder using the Mini-International Neuropsychiatric Interview-plus structured clinical interview, which is based on the DSM-IV. Among those diagnosed with bipolar I disorder, their first-degree relatives (parents, siblings, and children) who were aged 18 to 50 years and had education level of at least eighth grade were included. Individuals were excluded if they had a history of any psychiatric illness, learning disability, substance dependence, concurrent neurological illness, systemic illness known to cause cognitive impairment, head injury with loss of consciousness, or any medication known to impair cognition in the previous month. Additionally, healthy controls matched for age, sex, intelligence, and education level, with same exclusion criteria and absence of family history of psychiatric illness were recruited from the general population.
Sociodemographic data were collected using a semi- structured proforma. Participants were assessed for verbal and visual working memory, executive function (including cognitive flexibility, response inhibition, as well as concept formation, abstract reasoning, and set-shifting abilities), and affective temperament by a single investigator.
Intelligence was assessed using Raven’s progressive matrices,16 which is a standardised test comprising visually presented geometric analogy–like problems. The problems are a matrix of geometric figures with one entry missing, and the correct missing entry needs to be chosen from a list of possible answers. A score between the 25th and 75th percentiles is defined as intellectually average. Participants who scored at the 25th percentile or higher were included.
Verbal working memory was assessed using the N-back test (1-back and 2-back tests),17 in which 30 randomly ordered consonants common to multiple Indian languages are presented auditorily at the rate of one per second. Of these, nine consonants are randomly repeated. In the 1-back test, the participant responds whenever a consonant is repeated consecutively, whereas in the 2-back test, the participant responds whenever a consonant is repeated after an intervening consonant. The number of hits and errors are scored. The total number of errors is used to compute the score.
Visual working memory was assessed using the N-back test (1-back test),17 in which a black dot is placed randomly along the perimeter of an imagined circle on 36 cards. The dimension and location of the imaginary circle remains constant in all cards. Each card is presented individually. The participant is asked to respond whenever the location of the dot is repeated consecutively. The number of hits and errors is scored. The total number of errors is used to compute the score.
Cognitive flexibility was assessed using the Trail Making Test part B,18 in which 25 circles with both numbers (1-13) and letters (A-L) are distributed on a sheet of paper. Participants were asked to connect the 25 circles in an ascending order with alternation between numbers and letters (ie, 1-A-2-B-3-C, etc) as quickly as possible, without lifting the pen or pencil from the paper; errors were pointed out immediately and correction allowed. The time taken to complete the trail is recorded.
Response inhibition was assessed using the Stroop test,19 in which three cards with 20 rows and 5 columns of either colour names or symbols are presented. The first card has names of colours printed in black, the second card has symbols printed in different colours, and the last card has names of colours printed in different colours (eg, ‘red’ printed in green colour). Participants are asked to read the names of colours in the first card, and then name the colours of the symbols in the second card, and lastly identify the names of the colours printed in different colours in the third card. The times taken to read each card (t1, t2, t3) and the number of errors are recorded. The Stroop effect is calculated as: t3 − (t1 + t2/2).
Concept formation, abstract reasoning, and set-shifting abilities were assessed using the Wisconsin Card Sorting Test,20 which comprises 64 test cards and four stimulus cards that vary by colour (red, green, yellow, blue), form (triangle, star, cross, circle), and number (1, 2, 3, 4). The four stimulus cards are placed in front of the participant (from left to right: a card with a red triangle on the left, followed by a card with two green stars, a card with three yellow crosses, and a card with four blue circles). The participant matches each of the 64 test cards to one of the stimulus cards and is told only whether the response is correct or incorrect, without revealing the sorting principle. A correct match gets a score of 1; after ten consecutive correct matches, the examiner changes the sorting principle (colour, then form, and finally number) without informing the participant. Scores in terms of errors, perseverative responses, perseverative errors, non-perseverative errors, conceptual level responses, and categories completed were calculated.
Affective temperament was assessed using the short version of the Temperament Evaluation of Memphis, Pisa, Paris and San Diego Auto-questionnaire,21 which comprises five subscales: cyclothymic (12 questions), depressive (8 questions), irritable (8 questions), hyperthymic (8 questions), and anxious (3 questions); answers are either yes or no, and the number of yes responses for each subscale was recorded.
Data analysis was performed using SPSS (Windows version 20.0; IBM Corp, Armonk [NY], United States). The two groups were compared using the Chi-squared test or Mann-Whitney U test, as appropriate. A p value of <0.05 was considered statistically significant.
Results
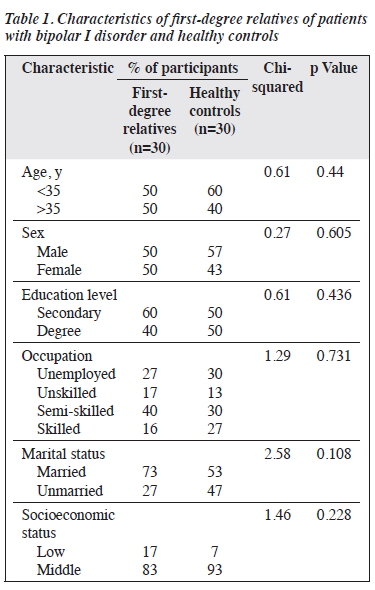
Of the 52 first-degree relatives of patients diagnosed with bipolar I disorder, 30 were included in the analysis and the remaining 22 were excluded owing to diagnoses of major depressive disorder (n=4), seizure disorder (active use of antiseizure medications) [n=3], alcohol dependence syndrome (n=13), or cannabis dependence syndrome (n=2). Additionally, 30 matched healthy controls from the general population were included for comparison.
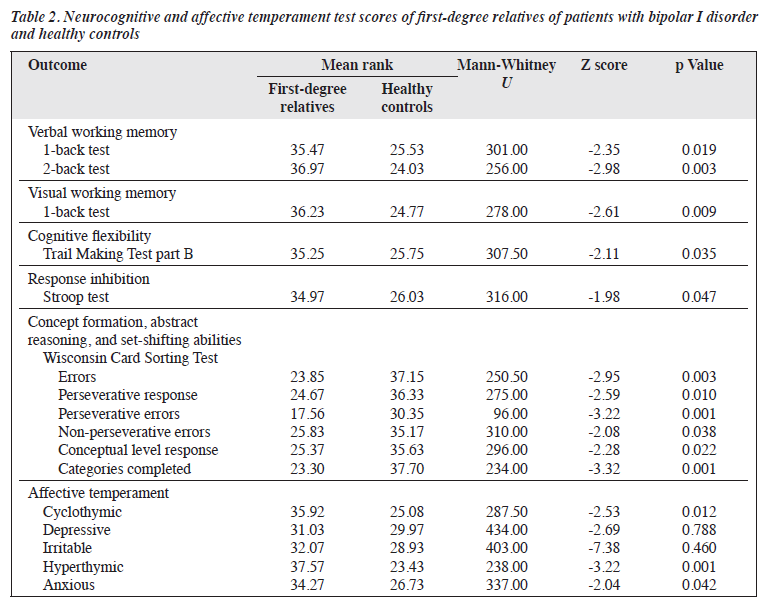
Cases and controls were comparable in terms of age, sex, education level, occupation, marital status, and socioeconomic status (Table 1). Both groups were predominantly of middle socioeconomic background. Compared with healthy controls, first-degree relatives performed significantly poorer in all tests and had significantly higher scores for cyclothymic, hyperthymic, and anxious temperaments (Table 2).
Discussion
Compared with healthy controls, first-degree relatives of patients with bipolar I disorder performed significantly poorer in various neurocognitive tests and had certain affective temperaments more frequently. These are prerequisites for establishing an endophenotype. First- degree relatives performed significantly poorer on the verbal 1-back and 2-back tests and visual 1-back test; this indicates deficits in verbal and visual working memory. Similar deficits in unaffected relatives of bipolar disorder probands have been reported.9,10,22,23 However, other studies have found no verbal or visual working memory impairment in such relatives.24-27 This discordance may be explained by the use of different neurocognitive tools and small sample sizes.
First-degree relatives showed significant impairment in cognitive flexibility as measured by the Trail Making Test part B, consistent with other studies.22,26,28 However, a recent study reported no difference in executive function between unaffected siblings of patients with bipolar I disorder and healthy controls.29 This could be because the study group comprised only unaffected siblings of patients with bipolar I disorder, whereas our study included unaffected parents and children as well as siblings.
Impaired response inhibition was also observed in first-degree relatives, consistent with other studies.22,30 In a meta-analysis, the most consistent marker for endophenotype in bipolar disorder was a deficit in response inhibition.24 However, other studies, despite with smaller sample sizes, have demonstrated no difference in performance on the Stroop test between unaffected relatives of bipolar probands and healthy controls.9,28
The first-degree relatives had more errors, especially perseverative errors, on the Wisconsin Card Sorting Test; this indicates impaired set shifting. The conceptual level responses and the total number of categories completed were lower; this indicates poorer concept formation, planning, and problem-solving skills. Executive dysfunction in unaffected relatives of bipolar probands has been demonstrated.10,27,31 A 16-month follow-up study also found stable deficits in working memory and executive function in unaffected relatives of patients with bipolar disorder over time.32 However, other studies have found no significant impairment in Wisconsin Card Sorting Test performance in healthy biological relatives of patients with bipolar disorder25,33; this may be explained by the ethnic diversity of the population.
First-degree relatives demonstrated significantly higher occurrence for cyclothymic, hyperthymic, and anxious temperaments, consistent with other studies.34-38 Temperament dysregulation is the primary abnormality that predisposes individuals to bipolar disorders; greater temperament variability is associated with increased risk and severity of bipolar disorder.39,40 This suggests that temperament is influenced by multiple small-effect genes, leading to a continuous spectrum of mood regulation. This aligns with observations of milder bipolar traits in relatives of patients with bipolar disorder and supports a polygenic transmission mode, making temperament a key tool in studying the genetics of bipolar disorder.39,40
Our study has some limitations. The sample size was small, and some variables were not normally distributed. The short version of the Temperament Evaluation of Memphis, Pisa, Paris and San Diego Auto-questionnaire was not available in any local language, requiring translation from English to local language and then back-translation to English for comparison with the original scale; this meant that the scale was not validated in Indian populations.
However, the study has several strengths. Among parents, siblings, and children of patients with bipolar I disorder, only those aged 18 to 50 years were included to avoid any age-related cognitive impairments, as were only those with education level of at least eighth grade and with an intelligence quotient of >70 (ie, a score of >25th percentile in Raven’s progressive matrices). Participants in case and control groups underwent structured clinical interview to exclude those with any other psychiatric illness or history of any neurological illness or substance dependence. The two groups were comparable in terms of age, sex, education level, occupation, and socioeconomic level. A broad battery of validated tests was used to evaluate outcome measures. This is one of a few studies conducted on a southern Indian sample.
Studies with a larger sample size and more diversity of age and intelligence are warranted to investigate if these factors have any effect on cognitive performance. Differences among parents, siblings, and children of bipolar probands should be investigated to determine the severity of impairment in each group. Studies including unaffected relatives of patients with various bipolar spectrum disorders, schizophrenia, or depression are also warranted, because endophenotype markers of working memory, executive function, and affective temperament have been demonstrated in patients with these disorders.
First-degree relatives are often the carers of patients with bipolar disorder. Identifying individuals at higher risk of developing bipolar disorder and monitoring for subclinical mood disturbances may facilitate timely intervention and improve long-term outcomes. Understanding the endophenotypes of bipolar disorder can aid in genetic counselling, education of family members, and encouraging proactive mental health measures such as lifestyle modification, stress management, and seeking early treatment. Endophenotypes of bipolar disorder may be targets for new medications or clinical trials because of their association with the pathophysiology.
Conclusion
Impairments in working memory, executive function, and certain affective temperaments are potential endophenotypes for bipolar I disorder. Working memory and executive function are most important cognitive domains for social, occupational, and interpersonal functioning. These potential markers could be used to trace susceptible genes for bipolar disorder and thus enhance our understanding of the complex genetics of mood disorders.
Contributors
TL designed the study, acquired the data, analysed the data, drafted the manuscript, and critically revised the manuscript for important intellectual content. BR and AS designed the study, drafted the manuscript, and critically revised the manuscript for important intellectual content. PDA, AMR, and RK analysed the data, drafted the manuscript, and critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding / support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data availability
All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics approval
This study was approved by the Institutional Ethics
Committee of Madras Medical College (reference: 34072012). The participants provided written informed consent for all treatments and procedures and for publication.
References
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry 2003;160:636-45.
- Hall MH, Smoller JW. A new role for endophenotypes in the GWAS era: functional characterization of risk variants. Harv Rev Psychiatry 2010;18:67-74.
- Myles-Worsley M, Coon H. Genetic and developmental factors in spontaneous selective attention: a study of normal twins. Psychiatry Res 1997;71:163-74.
- Anokhin AP, Heath AC, Ralano A. Genetic influences on frontal brain function: WCST performance in twins. Neuroreport 2003;14:1975-8.
- Ando J, Ono Y, Wright MJ. Genetic structure of spatial and verbal working memory. Behav Genet 2001;31:615-24.
- Quraishi S, Frangou S. Neuropsychology of bipolar disorder: a review. J Affect Disord 2002;72:209-26.
- van Gorp WG, Altshuler L, Theberge DC, Wilkins J, Dixon W. Cognitive impairment in euthymic bipolar patients with and without prior alcohol dependence. A preliminary study. Arch Gen Psychiatry 1998;55:41-6.
- Taj M, Padmavati R. Neuropsychological impairment in bipolar affective disorder. Indian J Psychiatry 2005;47:48-50.
- Ferrier IN, Chowdhury R, Thompson JM, Watson S, Young AH. Neurocognitive function in unaffected first-degree relatives of patients with bipolar disorder: a preliminary report. Bipolar Disord 2004;6:319- 22.
- Bora E, Vahip S, Akdeniz F, Ilerisoy H, Aldemir E, Alkan M. Executive and verbal working memory dysfunction in first-degree relatives of patients with bipolar disorder. Psychiatry Res 2008;161:318-24.
- Gonda X, Torok D, Eszlari N, et al. “… wise, amazed, temp’rate, and furious, Loyal and neutral, in a moment”: first heritability analysis of affective temperaments reports remarkably high SNP-based heritability. Eur Psychiatry 2023;66(Suppl 1):S352-S353.
- Goldsmith HH, Buss KA, Lemery KS. Toddler and childhood temperament: expanded content, stronger genetic evidence, new evidence for the importance of environment. Dev Psychol 1997;33:891- 905.
- Evans L, Akiskal HS, Keck PE, et al. Familiality of temperament in bipolar disorder: support for a genetic spectrum. J Affect Disord 2005;85:153-68.
- Kesebir S, Vahip S, Akdeniz F, Yüncü Z, Alkan M, Akiskal H. Affective temperaments as measured by TEMPS-A in patients with bipolar I disorder and their first-degree relatives: a controlled study. J Affect Disord 2005;85:127-33.
- Maier W, Minges J, Lichtermann D, Franke P, Gansicke M. Personality patterns in subjects at risk for affective disorders. Psychopathology 1995;28 Suppl 1:59-72.
- Raven J, Raven JC, Court JH. Manual for Raven’s Progressive Matrices and Vocabulary Scales. Oxford Psychologists Press; 1998: 73.
- Rao SL. NIMHANS Neuropsychology Battery-2004, Manual. National Institute of Mental Health and Neurosciences; 2004: 267.
- Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. Neuropsychology Press; 1985: 516.
- Lezak MD. Neuropsychological Assessment. Oxford University Press; 1995: 1048.
- Heaton R, Chelune C, Talley J, Kay G, Curtiss G. Wisconsin Card Sorting Test Manual – Revised and Expanded. 1993.
- Akiskal HS, Mendlowicz MV, Jean-Louis G, et al. TEMPS-A: validation of a short version of a self-rated instrument designed to measure variations in temperament. J Affect Disord 2005;85:45-52.
- Arts B, Jabben N, Krabbendam L, van Os J. Meta-analyses of cognitive functioning in euthymic bipolar patients and their first-degree relatives. Psychol Med 2008;38:771-85.
- Nehra R, Grover S, Sharma S, Sharma A, Sarkar S. Neuro-cognitive functioning in unaffected siblings of patients with bipolar disorder: comparison with bipolar patients and healthy controls. Indian J Psychiatry 2014;56:283-8.
- Bora E, Yucel M, Pantelis C. Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. J Affect Disord 2009;113:1- 20.
- Keri S, Kelemen O, Benedek G, Janka Z. Different trait markers for schizophrenia and bipolar disorder: a neurocognitive approach. Psychol Med 2001;31:915-22.
- Antila M, Tuulio-Henriksson A, Kieseppä T, Eerola M, Partonen T, Lönnqvist J. Cognitive functioning in patients with familial bipolar I disorder and their unaffected relatives. Psychol Med 2007;37:679-87.
- Trivedi JK, Goel D, Dhyani M, et al. Neurocognition in first-degree healthy relatives (siblings) of bipolar affective disorder patients. Psychiatry Clin Neurosci 2008;62:190-6.
- Pattanayak RD, Sagar R, Mehta M. Neurocognition in unaffected first- degree relatives of patients with bipolar disorder type I From India: a potential vulnerability marker? Sage Open 2012;2:1-6.
- Chandrasekaran V, Kattimani S, Subramanian K, Penchilaiya V, Karunanithi A. Cognitive performance and psychosocial functioning in unaffected siblings of bipolar disorder patients in comparison with healthy controls. Asian J Psychiatr 2020;54:102246.
- Zalla T, Joyce C, Szöke A, et al. Executive dysfunctions as potential markers of familial vulnerability to bipolar disorder and schizophrenia. Psychiatry Res 2004;121:207-17.
- Gillissie ES, Krupski JR, Jawad MY, et al. Evaluating cognitive function in unaffected relatives of individuals with bipolar disorders: a meta-analysis. J Psychiatr Res 2022;152:289-95.
- Kjærstad HL, Søhol K, Vinberg M, Kessing LV, Miskowiak KW. The trajectory of emotional and non-emotional cognitive function in newly diagnosed patients with bipolar disorder and their unaffected relatives: a 16-month follow-up study. Eur Neuropsychopharmacol 2023;67:4- 21.
- Frangou S, Haldane M, Roddy D, Kumari V. Evidence for deficit in tasks of ventral, but not dorsal, prefrontal executive function as an endophenotypic marker for bipolar disorder. Biol Psychiatry 2005;58:838-9.
- Chiaroni P, Hantouche EG, Gouvernet J, Azorin JM, Akiskal HS. The cyclothymic temperament in healthy controls and familially at risk individuals for mood disorder: endophenotype for genetic studies? J Affect Disord 2005;85:135-45.
- Mendlowicz MV, Jean-Louis G, Kelsoe JR, Akiskal HS. A comparison of recovered bipolar patients, healthy relatives of bipolar probands, and normal controls using the short TEMPS-A. J Affect Disord 2005;85:147-51.
- Saguem BN, Mtiraoui A, Nakhli J, et al. Affective temperaments and their relationships with life events in bipolar patients and siblings: a controlled study. J Ment Health 2021;30:36-42.
- Gandotra S, Ram D, Kour J, Praharaj SK. Association between affective temperaments and bipolar spectrum disorders: preliminary perspectives from a controlled family study. Psychopathology 2011;44:216-24.
- Mahon K, Perez-Rodriguez MM, Gunawardane N, Burdick KE. Dimensional endophenotypes in bipolar disorder: affective dysregulation and psychosis proneness. J Affect Disord 2013;151:695- 701.
- Akiskal HS. Toward a temperament-based approach to depression: implications for neurobiologic research. Adv Biochem Psychopharmacol 1995;49:99-112.
- Akiskal HS. The temperamental foundations of affective disorders. In: Mundt C, Goldstein MJ, Hahlweg K, Fiedler P, editors. Interpersonal Factors in the Origin and Course of Affective Disorders. London: Gaskell/Royal College of Psychiatrists; 1996: 3-30.