Hong Kong J Psychiatry 2004;14(3):2-6
ORIGINAL ARTICLE
Part of the findings were presented at the 11th Biennial Winter Workshop on Schizophrenia at Davos, Switzerland, 24 February– 1 March 2002.
Dr RCK Chan, PhD, Department of Psychology, Sun Yat-Sen University, Guangzhou, China, and Department of Psychiatry, The University of Hong Kong, Hong Kong, China.
Dr EYH Chen, MBChB, MD, MRCPsych(UK), FHKAM (Psych), Department of Psychiatry, The University of Hong Kong, Hong Kong, China.
Address for correspondence: Dr RCK Chan, Neuropsychology and Applied Cognitive Neuroscience Laboratory, Department of Psychology, Sun Yat-Sen University, Guangzhou 510275, China. Tel: (86 20) 8411 4267; Fax: (86 20) 8411 4266; E-mail: edschchq@zsu.edu.cn or rckchan@hkucc.hku.hk
Submitted: 24 April 2004; Accepted: 18 October 2004
Abstract
Objective: To further explore the relationship between a set of executive function factor scores and neurological features in patients with chronic schizophrenia.
Patients and Methods: This cross-sectional study investigated the Sustained Attention Response to Task, Six Elements Test, Hayling Sentence Completion Test, and Cambridge Neurological Inventory in 51 patients with schizophrenia.
Results: Significant relationships were found between executive function factors and neuro-logical signs after adjustment for the confounding effects of age, education, illness duration, and medication.
Conclusion: The derived factors seem to share common neural substrates with neurological soft signs in schizophrenia. Neurological soft signs could be adopted by clinicians as a bedside screening test of executive dysfunctions.
Key words: Dementia, Neurological manifestations, Schizophrenia
Introduction
Schizophrenia is a brain disorder affecting subjective experiences as well as a number of cognitive functions. Experimental and clinical studies adopting both cross-sectional1-3 and longitudinal designs4-5 also demonstrate that specific domains are differentially affected against a background of diffuse impairment in addition to a global decline in intellectual functioning.6 Consistent evidence of the major involvement of the frontal lobes in schizophrenia has been shown in a wide range of studies.7-12 These studies suggest that the frontal lobe, particularly the prefrontal region, is one of the main sites of abnormality in schizo-phrenia. Damage in this region has been shown to be asso-ciated with poor planning and mental rigidity,13 impaired social judgement,14 and impulsivity15 collectively known as dysexecutive syndrome.16 Impairments in executive functions are most obvious as patients attempt to cope with the complexities, open-ended situations, and social ambiguities of everyday life.
Recent research into executive function performance in clinical groups has emphasised the application of the ‘new generation tests’, which are primarily derived from theory of executive functions. The supervisory attention system17,18 is one of the most frequently used models for explaining cognitive disorders in patients with frontal lobe lesions,17-19 normal healthy adults,20 and clinical symptoms in schizo-phrenia.21 The supervisory attention system plays a major role in regulating the non-routine and novel task performance in daily life. Impairment of this system would be expected to result in a wide range of deficits in goal setting, planning, and making decisions between alternative sequences of behaviour in order to reach a particular goal.
The crucial role of neurological abnormalities or signs in schizophrenia has been recognised by Tsuang and Faraone as the ‘target features’ that encompass the idea that genetic and non-genetic processes lead to maldevelopment in neurocognitive systems.22 Target features should be increased in relatives of patients but perhaps not to a similar extent. In addition, the manifestation of multiple genes of small effect would lead to an expectation that target features should be present, to a lesser extent, in the general population. Neurological signs, therefore, also represent a potential intermediate phenotype in schizophrenia.23 In particular, the relationship between neurological soft signs and neuro-psychological performance deficits in schizophrenia have recently been gaining more attention. The term ‘soft signs’ is used in contrast to ‘hard signs’, which are localisable to specific brain regions and typically involve motor or perceptual systems. The boundary between neuropsychol-ogy and neurological signs is not sharp and uncontentious. It was not until recently that a few studies began to explore the relationships between specific domains of neurological signs and executive functions.24-26 In general, the frontal signs or soft neurological signs (motor coordination, sensory integration, and disinhibition) were significantly correlated with various neuropsychological dysfunctions. Wong et al found that the frontal signs were associated with random errors of the executive functions.26 Ross et al also found that the sensory integration item was the most frequent pre-dictor of eye-tracking performance in patients with chronic schizophrenia.25 These studies demonstrated that the fron-tal signs were the most discriminatory items in classifying these patients. Chen et al demonstrated that a frontally based attention task was strongly correlated with motor coordina-tion and disinhibition but not the other signs in a group of patients with chronic schizophrenia.27
Most recently, Chan et alsuccessfully demonstrated the construct validity of a set of executive function tests based on the supervisory attention system in patients with schizophrenia.28 Three factors were identified within the executive function tests, as follows:
- the ‘semantic inhibition factor’ comprised items inhi-biting verbal and semantic responses
- the ‘action/attention inhibition’ factor comprised items on error commission and rule-breaking score
- the ‘output generation factor’ comprised items on ini-tiation and generation of response.
This brief report further discusses the relationship between these derived factors and neurological signs in a group of patients with schizophrenia and suggests that frontally based tasks might functionally underlie the mani-festation of specific neurological soft signs in schizophrenia.
Patients and Methods
Fifty one inpatients with chronic schizophrenia (47 men and 4 women) were recruited at a regional psychiatric hospital. All patients met the Diagnostic and Statistical Manual of Mental Disorders-IV criteria for schizophrenia. Informed consent was obtained from all patients prior to the testing session in accordance with the Declaration of Helsinki. The mean age was 44 years (standard deviation [SD], 9.58 years) and the educational level was 8.2 years (SD, 2.86 years). The mean illness duration was 21.3 years (SD, 9.5 years). The mean daily antipsychotic drug dose was 1263.13 mg chlorpromazine equivalents (SD, 1577.74 mg; range, 700.00 to 7143.00 mg). All participants were right-handed according to the Edinburgh Inventory.29 The Positive and Negative Syndrome Scale (PANSS) was used for the as-sessment of symptoms.30 All items were rated from 1 (absent) to 7 (extreme) according to the standardised instructions. Inter-rater reliability for the PANSS was evaluated with the intra-class correlation coefficient (ICC). ICC was 0.83 for the global PANSS score, 0.84 for the positive symptoms subscale, and 0.73 for the negative symptoms subscale.
The details of the executive function tests selected for the study are described elsewhere.28 The tests used were the Sustained Attention Response to Task (SART),31 the Six Elements Test (SET),32 and the Hayling Sentence Comple-tion Test (HSC).19 The applicability of these measures has also been validated in the Hong Kong setting.20,28,33,34 Background cognition tests, including the short-form of the Wechsler Adult Intelligence Test-III35 and the Logical Memory and Visual Reproduction Tests of the Wechsler Memory Test-III36 were administered to all participants before the neuropsychological session. Table 1 summarises the qualitative features of these tests.
Neurological signs were examined using the Cambridge Neurological Inventory (CNI).37 The CNI offered standardised procedures for rating neurological signs in 7 subgroups according to the nature of individual items of these signs. These are ‘motor coordination’ (e.g., finger/ thumb opposition), ‘sensory integration’ (e.g., finger agno-sia and stereoagnosis), ‘extrapyramidal’ (e.g., glabellar signs and neck rigidity), ‘dyskinesia’ (e.g., trunk limb dyskinesia and orofacial dyskinesia), ‘catatonia’ (e.g., gait mannerisms and perseveration), ‘disinhibition’ (e.g., blinking during sac-cadic eye movements and go-nogo test), and ‘pyramidal’ (e.g., hyper- and hyporeflexia). In the original ratings scale, scoring was done according to standardised anchor points to indicate a ‘normal’ response (0), an ‘equivocal’ response (0.5), an ‘abnormal’ response (1.0), or a ‘grossly abnormal’ response (2.0). In the present study, item scores were further abbreviated into either absent (0; covering normal or equivo-cal scale scores) or present (1; covering abnormal or grossly abnormal scale scores). Inter-rater reliability was established by rating a sample of 15 of the patients in this study. The overall intra-class correlation coefficient was 0.94.
The composite scores of the derived factors28 were first computed by adding the standardised scores of the corre-sponding factor and were used for subsequent data analysis. Pearson product-moment correlations, controlling for age, education, duration of illness, and medication, were con-ducted to investigate the relationship between the factor composite scores and subscales of the neurological signs. For the analysis of the potential involvement of the execu-tive function in the manifestation of soft neurological signs, a series of multiple linear regression analyses were conducted. Individual neurological sign scores were treated as the dependent variables and neuropsychological domain scores were treated as the independent variables.
Results
There were significant correlations between age and motor coordination (r = 0.318; p = 0.023), sensory integration (r = 0.347; p = 0.013), logical memory immediate recall (r = -0.289; p = 0.04), visual reproduction immediate recall (r = -0.383; p = 0.006), and visual reproduction delayed recall (r = -0.316; p = 0.024). However, for educational levels, a significant correlation was found only in verbal performance (r = 0.398; p = 0.012). The duration of illness was also correlated with sensory integration (r = 0.336; p = 0.017), extrapyramidal signs (r = 0.308; p = 0.029), and catatonia (r = 0.303; p = 0.032).
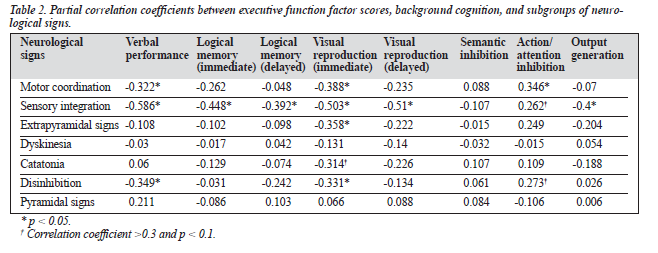
Partial correlation analyses, controlling for age, education, illness duration, and medication, revealed a series of significant correlations between the executive func-tion factor scores, different subscales of neurological signs, and background cognition. Table 2 indicates that, among the 7 subgroups of neurological signs, the action/attention inhibition factor was significantly correlated with motor coordination (r = 0.346; p = 0.023). There was also a trend for correlation between this factor and sensory integration (r = 0.262; p = 0.09) and disinhibition (r = 0.273; p = 0.077). Significant inverse correlations were found between the output generation factor and sensory integration (r = -0.4; p = 0.008).
Significant inverse correlations were also found between visual reproduction and motor coordination (r = -0.388; p = 0.021), sensory integration (r = -0.503; p = 0.002), disinhibition (r = -0.331; p = 0.05), and extrapyramidal signs (r = -0.358; p = 0.035). Logical memory was also corre-lated with sensory integration (r = -0.448; p = 0.007). Finally, verbal performance was inversely correlated with motor coordination (r = -0.322; p = 0.05), sensory integration (r = -0.586; p = 0.0005), and disinhibition (r = -0.349; p = 0.04).
The relationships between executive function factors and individual neurological soft signs (motor coordination, sen-sory integration, and disinhibition) were further explored. For motor coordination, the action inhibition factor was correlated with finger-thumb tapping for the left side (r = 0.26; p = 0.05), fist/edge/palm for the right side (r = 0.317; p = 0.038), and the Ozeretski test (r = 0.368; p = 0.015). For sensory integration, the semantic inhibition factor was inversely correlated with finger agnosia for the right side (r = -0.31; p = 0.04). The action/attention inhibition factor was correlated with extinction (r = 0.442; p = 0.003) and left/right orientation (r = 0.355; p = 0.019) and inversely correlated with graphaesthesia for the left side (r = -0.271; p = 0.05). The output generation factor was inversely correlated with finger agnosia for the right side (r = -0.329; p = 0.031), left graphaesthesia (r = -0.269; p = 0.05), and left/right orientation (r = -0.315; p = 0.04). For disinhibition, the action inhibition factor was significantly correlated with the go-nogo item (r = 0.377; p = 0.013).
For neurological signs as dependent variables, signifi-cant neurocognitive contributors to motor coordination signs included the action/attention inhibition score and the memory composite score [F(2,32) = 5.706; p = 0.008]. Significant contributors to sensory integration signs included only the memory composite score [F(1,33) = 14.364; p = 0.0006]. The neurocognitive function and executive functions were not found to be predictive of disinhibition signs and other hard neurological signs.
Discussion
These findings indicate that significant but modest relation-ships were differentially demonstrated among these variables, taking into account age, education, and the dura-tion of illness, as well as medication. Among these neuro-logical signs, motor coordination, sensory integration, and disinhibition signs have traditionally been regarded as soft neurological signs, largely on conceptual and theoretical bases.38 In contrast with other groups of hard neurological signs, we have found that soft neurological signs are more cohesively related to one another and are particularly closely related to impairments in the executive functions. However, no significant correlation was found among the executive function factors and other hard neurological signs such as extrapyramidal and catatonic signs. These findings are con-sistent with previous studies26,39 — namely, that the neuro-logical signs could be subclassified into frontal signs and soft neurological signs. Importantly, the relationship with cognitive deficits appears to be specific and different for 2 subgroups of soft neurological signs (motor coordination and sensory integration). These results therefore provide some empirical support for the classification of soft neuro-logical signs into motor coordination and sensory integra-tion subgroups.40
Motor coordination signs were specifically associated with impairments in action and attention inhibition, as well as with verbal performance and visual memory. It is note-worthy that verbal performance has also been related to general intelligence and executive function, and it has been sug-gested that frontal lobe function is critically involved.41 In this context, it is not surprising that motor coordination signs were significantly related to both verbal performance and executive function components. Sensory integration signs were generally related to a wider range of neurocognitive functions in addition to executive functions. In schizo-phrenia, there is evidence for a generalised cognitive decline, which affects performance in verbal functioning.42 The extremely strong association between sensory integration and verbal performance suggests that the presence of the latter is probably a reflection of a generalised cognitive impairment. The present data also suggest that dis-inhibition signs are also specifically related to the action/ attention inhibition component of the executive functions. Item by item analysis revealed a specific relationship be-tween the go-nogo test and the action/attention inhibition factor of executive function.
This brief report is limited by a number of methodologi-cal issues. In light of the total number of correlations, the significant correlations found between the executive function factors and neurological signs might warrant multiple group comparison adjustment. However, in view of the ex-ploratory nature of this report, it is still worthwhile and rel-evant to the understanding of the underlying neural substrates between neurological signs and executive functions to a certain extent. Future study should recruit a larger sample with stringent statistical analysis controlling for multiple comparison adjustment. This study is one of very few stud-ies to explore the relationship between executive functions and neurological signs in schizophrenia using supervisory-attentional-based measures.
Acknowledgements
This project was supported in part by the Research Initia-tion Fund No. 10203555 from the University of Hong Kong to Dr Raymond Chan. The authors would also like to thank patients and staff at Castle Peak Hospital for their partici-pation and assistance in the study.
References
- Shallice T, Burgess PW, Frith CD. Can the neuropsychological case-study approach be applied to schizophrenia? Psychol Med 1991;21: 661-673.
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology 1998;12: 426-445.
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull 2000;26:119-136.
- Bilder RM, Degreef G, Pandurangi AK, Reider RO, Sakeim HA, Mukherjee S. Neuropsychological deterioration and CT scan findings in chronic schizophrenia. Schizophr Res 1988;1:37-45.
- Sweeney JA, Haas GL, Li S. Neuropsychological and eye movement abnormalities in first episode and chronic schizophrenia. Schizophr Bull 1992;18:283-293.
- Barber F, Pantelis C, Bodger S, Nelson HE. Intellectual functioning in schizophrenia: natural history. In: Pantelis C, Nelson HE, Barnes TRE, editors. Schizophrenia: a neuropsychological perspective. Chichester: John Wiley & Sons; 1996:49-70.
- Crow TJ, Ball J, Bloom SR, et al. Schizophrenia as an anomaly of development of cerebral asymmetry. Arch Gen Psychiatry 1989;46: 1145-1150.
- Gattaz WF, Kohlmeyer K, Gasser T. Computer tomographic studies in schizophrenia. In: Hafner H, Gattaz WF, editors. Search for the causes of schizophrenia. Vol II. Berlin: Springer; 1991:242-256.
- Weinberger DR. Schizophrenia and the frontal lobe. Trends Neurosci 1988;11:367-370.
- Sharpiro RM. Regional neuropathology in schizophrenia: where are we? Where are we going? Schizophr Res 1993;10:187-239.
- McKenna PJ, Chua S Schizophrenia: a brain disease? A critical re-view of structural and functional cerebral abnormality in the disorder. Br J Psychiatry 1995;166:563-582.
- Goldman-Rakic PS, Selemon, LD. Functional and anatomical aspects of prefrontal pathology in schizophrenia. Schizophr Bull 1997;23: 437-458.
- Benson DF, Miller BL. Frontal lobes: clinical and anatomic aspects. In: Feinberg TE, Farah MJ, editors. Behavioural neurology and neuropsychology. New York: McGraw-Hill; 1997:401-408.
- Damasio AR, Anderson SW. The frontal lobes. In: Heilman KM, Valenstein E, editors. Clinical neuropsychology. 3rd ed. New York: Oxford University Press; 1993:409-460.
- Stuss DT, Benson DF. Neuropsychological studies of the frontal lobes. Psychol Bull 1984;95:3-28.
- Baddeley A. Working memory. Oxford: Oxford University Press; 1986.
- Norman DA, Shallice T.Attention to action: willed and automatic con-trol of behaviour. In: Davidson RJ, Schwartz GE, Shapiro D, editors. Consciousness and self-regulation. Vol 4. New York: Plenum Press; 1986:1-18.
- Shallice T. From neuropsychology to mental structure. Cambridge: Cambridge University Press; 1988.
- Burgess PW, Shallice T. Response suppression, initiation and strategy use following frontal lose lesions. Neuropsychologia 1996;34:263-273.
- Chan RCK. Dysexecutive symptoms among a non-clinical sample: a study with the use of the dysexecutive questionnaire. Br J Psychology 2001;92:551-565.
- Frith CD. The cognitive neuropsychology of schizophrenia. Hove: Erlbaum; 1992.
- Tsuang MT, Faraone SV. The concept of target features in schizophre-nia research. Acta Psychiatr Scand 1999;395 (Suppl):2-11.
- Egan MF, Hyde TM, Bonomo JB, et al. Relative risk of neurological signs in siblings of patients with schizophrenia. Am J Psychiatry 2000; 158:1827-1834.
- Cuesta MJ, Peralta V, Zarzuela A, Calvo R, Garcia M, Serrano F. Neu-rological soft-signs in psychosis: threshold criteria for discriminating normal controls and for predicting cognitive impairment. Schizophr Res 2002;58:263-271.
- Ross DE, Buchanan RW, Medoff D, Lahti AC, Thaker GK. Associa-tion between eye tracking disorder in schizophrenia and poor sensory integration. Am J Psychiatry 1998;155:1352-1357.
- Wong AHC, Voruganti LNP, Heslegrave RJ. Neurocognitive deficits and neurological signs in schizophrenia. Schizophr Res 1997;23: 139-146.
- Chen EY, Lam LC, Chen RY, Nguyen DG, Kwok CL, Au UW. Neuro-logical signs and sustained attention impairment in schizophreni Eur Arch Psychiatry Clin Neurosci 2001;251:1-5.
- Chan RC, Chen EY, Cheung EF, Cheung, HK. Executive dysfunctions in schizophrenia: relationships to clinical manifestations. Eur Arch Psychiatry Clin Neurosci 2004;254:256-262.
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971;9:997-113.
- Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987;13:261-276.
- Robertson IH, Manly T, Andrade J, Baddeley BT, Yiend J. “Oops!”: Performance correlates of everyday attentional failures in traumatic brain injured and normal subjects. Neuropsychologia 1997;35:747-758.
- Wilson BA, Alderman N, Burgess PW, Emslie H, Evans JJ. Behavioural Assessment of the Dysexecutive Syndrome (BADS). Bury St Edmunds: Thames Valley Test Company; 1996.
- Chan RCK. Base-rate of postconcussion symptoms among non-clinical sample and its neuropsychological correlates. Clin Rehab 2001; 15:266-273.
- Chan RC, Manly T. The applicability of ‘dysexecutive syndrome’ mea-sures across cultures: performance and checklist assessment in neuro-logically healthy and traumatically brain-injured Chinese Hong Kong residents. J Int Neuropsychol Soc 2002;8:771-780.
- Wechsler, D. WAIS-III administration and scoring manual. San Antonio: The Psychological Corporation; 1997.
- Wechsler D. MS-III administration and scoring manual. San Antonio: The Psychological Corporation; 1997.
- Chen EYH, Shapleske J, Luque R, et al. The Cambridge Neurological Inventory: a clinical instrument for soft neurological signs and the fur-ther neurological examination for psychiatric patients. Psychiatry Res 1995;56:183-202.
- Heinrichs DW, Buchanan RW. Significance and meaning of neuro-logical signs in schizophrenia. Am J Psychiatry 1988;145:11-18.
- Arango C, Bartko JJ, Gold JM, Buchanan RW. Prediction of neuropsy-chological performance by neurological signs in schizophrenia. Am J Psychiatry 1999;156:1349-1357.
- Chen EY, Kwok CL, Au JW, Chen RY, Lau BS. Progressive deteriora-tion of soft neurological signs in chronic schizophrenic patients. Acta Psychiatr Scand 2000;102:342-349.
- Duncan J, Seitz RJ, Kolodny J, et al. A neural basis for general intelligence. Science 2000;289:457-460.
- Bilder RM, Lipschutz-Broch L, Reiter G, Geisler SH, Mayerhoff DL, Liberman JA. Intellectual deficits in first-episode schizophrenia: evi-dence for progressive deterioration. Schizophr Bull 1992;18:437-448.