East Asian Arch Psychiatry 2025;35:16-20 | https://doi.org/10.12809/eaap2502
ORIGINAL ARTICLE
Riski Syahna, Mustafa Mahmud Amin, Vita Camellia, Elmeida Effendy, Zulham Yamamoto
Abstract
Objectives: The efficacy of current schizophrenia treatments on cognitive symptoms remains limited owing to constrained data around the aetiopathogenesis of such symptoms. Complete blood cell counts have been used to identify variations in inflammatory responses among individuals with schizophrenia. This study aimed to determine associations between inflammatory markers and cognitive impairment in patients with schizophrenia.
Methods: Patients with schizophrenia aged 20 to 40 years were recruited from Prof Dr M Ildrem Hospital, Medan, Indonesia. Diagnoses were made by psychiatrists based on the DSM-5 criteria. Healthy controls matched for age, sex, and body mass index were recruited from the local community. The severity of schizophrenia was assessed by a psychiatrist using the Positive and Negative Syndrome Scale. Cognitive performance was assessed using the Montreal Cognitive Assessment (MoCA). Complete blood cell counts were performed. Absolute neutrophil, lymphocyte, monocyte, and white blood cell (WBC) counts were quantified, and the neutrophil-to-lymphocyte ratio (NLR) and monocyte-to-lymphocyte ratio (MLR) were calculated.
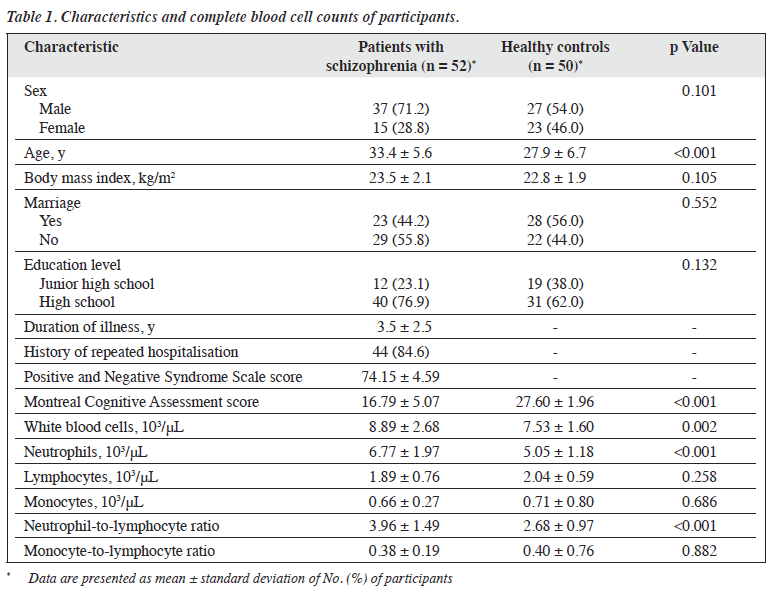
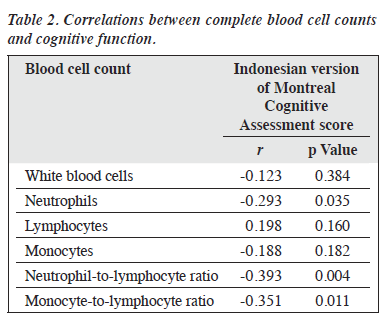
Results: In total, 64 men and 38 women were included in the analysis. Patients with schizophrenia (n = 52) and healthy controls (n = 50) were comparable in terms of all baseline characteristics, except that patients with schizophrenia were older (33.4 vs 27.9 years, p < 0.001) and had lower MoCA scores (16.79 vs 27.60, p < 0.001). For patients, the mean illness duration was 3.5 years, and the mean Positive and Negative Syndrome Scale score was 74.15. Patients with schizophrenia had higher WBC counts (8.89 vs 7.53 103/μL, p = 0.002), neutrophil counts (6.77 vs 5.05 103/μL, p < 0.001), and NLR (3.96 vs 2.68, p < 0.001). Neutrophil counts (r = -0.293, p = 0.035), NLR (r = -0.393, p = 0.004), and MLR (r = -0.351, p = 0.011) were negatively correlated with MoCA scores.
Conclusion: Patients with schizophrenia show signs of cognitive impairment and elevated WBC counts, neutrophil counts, and NLR. These peripheral inflammatory markers may be used to enhance understanding of the complex inflammatory theory of psychotic disorders.
Key words: Blood cell count; Cognition; Inflammation; Schizophrenia
Riski Syahna, Department of Psychiatry, Faculty of Medicine, Universitas Sumatera Utara, Medan, Indonesia
Mustafa Mahmud Amin, Department of Psychiatry, Faculty of Medicine, Universitas Sumatera Utara, Medan, Indonesia
Vita Camellia, Department of Psychiatry, Faculty of Medicine, Universitas Sumatera Utara, Medan, Indonesia
Elmeida Effendy, Department of Psychiatry, Faculty of Medicine, Universitas Sumatera Utara, Medan, Indonesia
Zulham Yamamoto, Department of Histology, Faculty of Medicine, Universitas Sumatera Utara, Medan, Indonesia
Address for correspondence: Dr Mustafa Mahmud Amin, Department of Psychiatry, Faculty of Medicine, Universitas Sumatera Utara, Medan, Indonesia. Email:mustafa.mahmud@usu.ac.id
Submitted: 3 January 2025; Accepted: 3 February 2025
Introduction
Schizophrenia is a severe and debilitating neuropsychiatric disorder. The positive, negative, and cognitive symptoms of schizophrenia all contribute to significant economic burden, particularly in middle-income countries.1-3 Cognitive symptoms can occur at any stage of schizophrenia and can lead to reduced quality of life.4 Approximately 80% of patients with schizophrenia have major impairments in social functioning, independent living, and occupation.5,6
Cognitive impairments in schizophrenia range from sensory and perceptual dysfunctions to higher-order cognitive dysfunctions such as executive function, working memory, attention-processing speed, verbal and visual learning, and memory.4,7-9 Deficits in cognitive function also significantly affect social functioning.
Recent genetic and epidemiological research suggests that the immune system may play a significant role in mediating cognitive symptoms.10-12 Current treatments for schizophrenia do not successfully address cognitive impairments, probably owing to constrained data around the pathogenesis of such symptoms. Aetiology and pathogenesis studies of cognitive impairment in schizophrenia have indicated a strong correlation between cognitive deficits and inflammation (and its various biochemical markers).5 However, no specific drug has yet been established as a targeted treatment option for cognitive symptoms.13
Biomarkers offer valuable insights into the aetiopathogenesis of diseases, identification of therapeutic targets, monitoring of patient progress, and prognosis. Peripheral inflammation can be assessed using complete blood cell counts, which include levels of neutrophils, lymphocytes, monocytes, and their ratios. Both the neutrophil-to-lymphocyte ratio (NLR) and the monocyte-to- lymphocyte ratio (MLR) represent systemic inflammation and, indirectly, neuroinflammation. They reflect correlations between innate immunity (neutrophils and monocytes) and adaptive immunity (lymphocytes) during illness and various pathological states.14 Both ratios are potential peripheral inflammatory indicators, especially for schizophrenia.15-18
The pathogenesis of schizophrenia includes immune dysregulation, which mainly causes cognitive deficits. Thus, it is important to assess changes in inflammatory markers and their associations with cognitive impairment in schizophrenia. Complete blood cell counts have been used to identify differences in inflammatory responses between individuals with and without schizophrenia. Therefore, this study aimed to determine associations between inflammatory markers and cognitive impairment in patients with schizophrenia.
Methods
This cross-sectional study was conducted between September and November 2024. Patients with schizophrenia aged 20 to 40 years were recruited from Prof Dr M Ildrem Hospital, Medan, Indonesia. Diagnoses were made by psychiatrists based on the third edition of the Indonesian Guidelines for Classification and Diagnosis of Mental Disorders and the DSM-5 criteria. Patients with obesity (body mass index ≥27 kg/m2) were excluded (because obesity affects cognitive function19), as were those with other mental disease, autoimmune disease, acute infection, chronic inflammation, or a history of antibiotic use in the past 2 weeks. Healthy controls matched for age, sex, and body mass index were recruited from the local community. Based on a previous study in China,8 an estimated sample size of 51 was required, assuming a 5% margin of error and a 0.05 significance level.
The severity of schizophrenia was assessed by a psychiatrist using the Indonesian version of the Positive and Negative Syndrome Scale (PANSS); scores of 60 to 80 indicate stable condition.20 Cognitive performance was assessed using the Indonesian version of the Montreal Cognitive Assessment (MoCA), which has been shown to be reliable and valid in Indonesian populations.21,22
Blood samples were taken in the morning prior to breakfast. Complete blood cell counts were performed using a Mindray BC-5000 Automated Hematology Analyzer (Mindray; Shenzhen, China). Absolute neutrophil, lymphocyte, monocyte, and white blood cell (WBC) counts were quantified, and the NLR and MLR were calculated.
Data were analysed using SPSS (Windows version 23.0; IBM Corp, Armonk [NY], United States). Normality of the data was assessed using the Kolmogorov-Smirnov test, and normally distributed data were evaluated using a two-tailed independent t test. Correlations between complete blood counts and cognitive performance were assessed using the Pearson correlation test. All tests were two-tailed; a p value of <0.05 was considered statistically significant.
Results
In total, 64 men and 38 women were included in the analysis (Table 1). Patients with schizophrenia (n = 52) and healthy controls (n = 50) were comparable in terms of all baseline characteristics, except that patients with schizophrenia were older (33.4 vs 27.9 years, p < 0.001) and had lower MoCA scores (16.79 vs 27.60, p < 0.001). For patients, the mean illness duration was 3.5 years, and the mean PANSS score was 74.15. Patients with schizophrenia had higher WBC counts (8.89 vs 7.53 103/μL, p = 0.002), neutrophil counts (6.77 vs 5.05 103/μL, p < 0.001), and NLR (3.96 vs 2.68, p < 0.001). Neutrophil counts (r = -0.293, p = 0.035), NLR (r = -0.393, p = 0.004), and MLR (r = -0.351, p = 0.011) were negatively correlated with MoCA scores (Table 2).
Discussion
Compared with healthy controls, patients with schizophrenia had higher levels of inflammatory markers including WBCs, neutrophils, and NLR, consistent with other studies.1,8,15,17,23-32 This suggests that inflammation may contribute to the aetiopathophysiology of schizophrenia via various pathways including oxidative stress, immune response, and neurodegenerative processes.33 WBCs such as neutrophils, lymphocytes, and monocytes are important components of the immune system. Leukocyte counts are significantly increased in patients with first-episode psychosis who have not received any antipsychotic therapy.24,34 In our patients, the mean duration of illness was 3.5 years, and most patients had experienced repeated episodes and received various antipsychotic medications. Thus, elevated WBC count, neutrophil count, and NLR in patients with schizophrenia may partially explain the increased risk of schizophrenia in at-risk individuals.
Compared with healthy controls, patients with schizophrenia had significantly poorer cognitive performance, which deteriorates progressively with increasing severity of the disease, consistent with other studies.8,18,33,35 Their neutrophil count, NLR, and MLR were also associated with reduced cognitive performance, consistent with other studies.8,17,18 NLR is negatively correlated with working memory,17 and then worsening cognitive function increases NLR.18 In patients with first-episode psychosis, neutrophil levels are negatively correlated with brain volume, which is associated with cognitive function.16 These findings demonstrate correlations between immune dysregulation and deficits in cognitive function in schizophrenia. The potential mechanism is that cognitive decline occurs gradually as a result of inflammatory responses to psychological or environmental stress. Given the unquantified variability in stress levels and the intricate correlations between inflammatory status and cognitive performance, these inflammatory markers should not be regarded as reliable markers to predict cognitive impairment during active states in first-episode untreated schizophrenia. Therefore, in our patients, their PANSS scores indicated stable condition and having undergone acute-phase treatment (to control for bias).
In patients with schizophrenia, MLR was negatively correlated with MoCA scores. The quantity of monocytes is an indirect indicator of microglial activation within the central nervous system.15 Monocytes may adversely impact brain structure and cognitive abilities in patients with schizophrenia.35 The presence of mononuclear lymphocytes in cerebrospinal fluid correlates with verbal fluency;36 the increase of these cells may be driven by the activation of microglia, resulting in cognitive deficits, particularly in terms of the speed of information processing.
There were several limitations to our study. Only complete blood cell counts were assessed; other inflammatory factors were not accounted for. Considering the intricacies of immune responses, our findings offer only a partial insight into the systemic inflammatory state. Nonetheless, chronic low-grade inflammation is potentially associated with certain aspects of cognitive impairment. Potential mechanisms of cognitive impairment in schizophrenia include activation of microglia, imbalance in monoamine levels, structural brain changes, and alterations in the kynurenine pathway.3 In addition, the effect of emotional conditions on inflammatory elements should have been assessed. Anxiety and depression, which those with schizophrenia may experience, are likely to affect the immune response.37 Additionally, tobacco use38 and antipsychotic medications39 can influence complete blood cell counts, but we did not collect such data. Consistent blood monitoring may offer a more comprehensive understanding of this matter.
Conclusion
Patients with schizophrenia show signs of cognitive impairment and elevated WBC counts, neutrophil counts, and NLR. These peripheral inflammatory markers may be used to enhance understanding of the complex inflammatory theory of psychotic disorders.
Contributors
All authors designed the study, acquired the data, analysed the data, drafted the manuscript, and critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding / support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data availability
All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics approval
This study was approved by the health research ethics committee of Universitas Sumatera Utara (reference: 1107/ KEPK/USU/2024). The participants provided written informed consent for all treatments and procedures and for publication.
References
- Gao Z, Li B, Guo X, Bai W, Kou C. The association between schizophrenia and white blood cells count: a bidirectional two-sample Mendelian randomization study. BMC Psychiatry 2023;23:271. Crossref
- Charlson FJ, Ferrari AJ, Santomauro DF, et al. Global epidemiology and burden of schizophrenia: findings from the Global Burden of Disease Study 2016. Schizophr Bull 2018;44:1195-203. Crossref
- Ribeiro-Santos A, Lucio Teixeira A, Salgado JV. Evidence for an immune role on cognition in schizophrenia: a systematic review. Curr Neuropharmacol 2014;12:273-80. Crossref
- McCutcheon RA, Reis Marques T, Howes OD. Schizophrenia: an overview. JAMA Psychiatry 2020;77:201-10. Crossref
- Bora E. Peripheral inflammatory and neurotrophic biomarkers of cognitive impairment in schizophrenia: a meta-analysis. Psychol Med 2019;49:1971-9. Crossref
- McCutcheon RA, Keefe RSE, McGuire PK. Cognitive impairment in schizophrenia: aetiology, pathophysiology, and treatment. Mol Psychiatry 2023;28:1902-18. Crossref
- Misiak B, Frydecka D, Stanczykiewicz B, Samochowiec J. Editorial: peripheral markers of immune response in major psychiatric disorders: where are we now and where do we want to be? Front Psychiatry 2019;10:5. Crossref
- Zhou L, Ma X, Wang W. Immune dysregulation is associated with symptom dimensions and cognitive deficits in schizophrenia: accessible evidence from complete blood count. BMC Psychiatry 2024;24:48. Crossref
- Patlola SR, Donohoe G, McKernan DP. The relationship between inflammatory biomarkers and cognitive dysfunction in patients with schizophrenia: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 2023;121:110668. Crossref
- Meyer U, Schwarz MJ, Müller N. Inflammatory processes in schizophrenia: a promising neuroimmunological target for the treatment of negative/cognitive symptoms and beyond. Pharmacol Ther 2011;132:96-110. Crossref
- 1 Tripathi A, Kar SK, Shukla R. Cognitive deficits in schizophrenia: understanding the biological correlates and remediation strategies. Clin Psychopharmacol Neurosci 2018;16:7-17. Crossref
- North HF, Bruggemann J, Cropley V, et al. Increased peripheral inflammation in schizophrenia associated with worse cognitive performance and related cortical thickness reductions. Eur Arch Psychiatry Clin Neurosci 2021;271:595-607. Crossref
- Cho M, Lee TY, Kwak YB, Yoon YB, Kim M, Kwon JS. Adjunctive use of anti-inflammatory drugs for schizophrenia: a meta-analytic investigation of randomized controlled trials. Aust N Z J Psychiatry 2019;53:742-59. Crossref
- Marazziti D, Torrigiani S, Carbone MG, et al. Neutrophil/lymphocyte, platelet/lymphocyte, and monocyte/lymphocyte ratios in mood disorders. Curr Med Chem 2022;29:5758-81. Crossref
- Mazza MG, Rossetti A, Clerici M. A review of neutrophil-lymphocyte, monocyte-lymphocyte, and platelet-lymphocyte ratios use in psychiatric disorders. World J Depress Anxiety 2018;1:1002.
- Núñez C, Stephan-Otto C, Usall J, et al. Neutrophil count is associated with reduced gray matter and enlarged ventricles in first-episode psychosis. Schizophr Bull 2019;45:846-58. Crossref
- Frota IJ, de Oliveira ALB, De Lima DN Jr, et al. Decrease in cognitive performance and increase of the neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios with higher doses of antipsychotics in women with schizophrenia: a cross-sectional study. BMC Psychiatry 2023;23:558. Crossref
- Liang J, Guan X, Sun Q, Hao Y, Xiu M. Neutrophil/lymphocyte ratio and cognitive performances in first-episode patients with schizophrenia and healthy controls. Prog Neuropsychopharmacol Biol Psychiatry 2024;135:111092. Crossref
- Dye L, Boyle NB, Champ C, Lawton C. The relationship between obesity and cognitive health and decline. Proc Nutr Soc 2017;76:443-54. Crossref
- Opler LA, Opler M, Malaspina D. Reducing guesswork in schizophrenia treatment. Curr Psychiatry 2006;5:76-84.
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695-9. Crossref
- Husein N, Lumempouw SF, Ramli Y, Herqutanto. Montreal Cognitive Assessment Versi Indonesia [in Indonesian]. Neurona 2010;27.
- Çatak Z, Uzmez E, Ozturk N, Ugur K. Comparison of neutrophil/ lymphocyte, platelet/lymphocyte and monocyte/lymphocyte ratios in patients with schizophrenia, bipolar, and major depressive disorder. Int J Med Biochem 2018;1:106-10. Crossref
- Jackson AJ, Miller BJ. Meta-analysis of total and differential white blood cell counts in schizophrenia. Acta Psychiatr Scand 2020;142:18-26. Crossref
- Zhu X, Zhou J, Zhu Y, et al. Neutrophil/lymphocyte, platelet/ lymphocyte and monocyte/lymphocyte ratios in schizophrenia. Australas Psychiatry 2022;30:95-9. Crossref
- Özdin S, Sarisoy G, Böke Ö. A comparison of the neutrophil- lymphocyte, platelet-lymphocyte and monocyte-lymphocyte ratios in schizophrenia and bipolar disorder patients: a retrospective file review. Nord J Psychiatry 2017;71:509-12. Crossref
- Orhan F, Schwieler L, Fatouros-Bergman H, et al. Increased number of monocytes and plasma levels of MCP-1 and YKL-40 in first-episode psychosis. Acta Psychiatr Scand 2018;138:432-40. Crossref
- Leung KK, Wong YC, Shea KS, et al. Altered neutrophil-to- lymphocyte ratio in patients with non-affective first episode psychosis and its relationship with symptom severity and cognitive impairment. Sci Rep 2023;13:11453. Crossref
- Gercek HG, Citir BG, Bukulme A. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as inflammation markers for early-onset schizophrenia. Bratisl Lek Listy 2023;124:503-7. Crossref
- Šagud M, Madžarac Z, Nedic Erjavec G, et al. The associations of neutrophil-lymphocyte, platelet-lymphocyte, monocyte-lymphocyte ratios and immune-inflammation index with negative symptoms in patients with schizophrenia. Biomolecules 2023;13:297. Crossref
- Karageorgiou V, Milas GP, Michopoulos I. Neutrophil-to-lymphocyte ratio in schizophrenia: a systematic review and meta-analysis. Schizophr Res 2019;206:4-12. Crossref
- Bioque M, Matias-Martins AC, Llorca-Bofí V, et al. Neutrophil to lymphocyte ratio in patients with a first episode of psychosis: a two- year longitudinal follow-up study. Schizophr Bull 2022;48:1327-35. Crossref
- İmre O, Caglayan C, Muştu M. The relationship of cognitive dysfunction with inflammatory markers and carotid intima media thickness in schizophrenia. J Pers Med 2023;13:1342. Crossref
- Yan J, Chen Y, Ju P, et al. Network association of biochemical and inflammatory abnormalities with psychiatric symptoms in first-episode schizophrenia patients. Front Psychiatry 2022;13:834539. Crossref
- Chen S, Fan F, Xuan FL, et al. Monocytic subsets impact cerebral cortex and cognition: differences between healthy subjects and patients with first-episode schizophrenia. Front Immunol 2022;13:900284. Crossref
- Snyder A, Grant H, Chou A, et al. Immune cell counts in cerebrospinal fluid predict cognitive function in aging and neurodegenerative disease. Alzheimers Dement 2023;19:3339-49. Crossref
- Sakrajda K, Szczepankiewicz A. Inflammation-related changes in mood disorders and the immunomodulatory role of lithium. Int J Mol Sci 2021;22:1532. Crossref
- Bioque M, Llorca-Bofí V, Salmerón S, et al. Association between neutrophil to lymphocyte ratio and inflammatory biomarkers in patients with a first episode of psychosis. J Psychiatr Res 2024;172:334-9. Crossref
- Zhang Y, Tao S, Coid J, et al. The role of total white blood cell count in antipsychotic treatment for patients with schizophrenia. Curr Neuropharmacol 2024;22:159-67. Crossref