East Asian Arch Psychiatry 2010;20:174-9
ORIGINAL ARTICLE
Mr Kevin KS Chan, MPhil, Department of Psychiatry, The University of Hong Kong, Hong Kong, China.
Dr Christy LM Hui, PhD, Department of Psychiatry, The University of Hong Kong, Hong Kong, China.
Dr May ML Lam, MBBS, MRCPsych, FHKCPsych, FHKAM (Psychiatry), Department of Psychiatry, The University of Hong Kong, Hong Kong, China.
Ms Jennifer YM Tang, BSocSc, Department of Psychiatry, The University of Hong Kong, Hong Kong, China.
Dr Gloria HY Wong, PhD, Department of Psychiatry, The University of Hong Kong, Hong Kong, China.
Dr Sherry KW Chan, MBBS, MRCPsych, MPhil, Department of Psychiatry, The University of Hong Kong, Hong Kong, China.
Prof Eric YH Chen, MD, MRCPsych, FHKCPsych, FHKAM (Psychiatry), Department of Psychiatry, The University of Hong Kong, Hong Kong, China.
Address for correspondence: Prof Eric YH Chen, Department of Psychiatry, The University of Hong Kong, Queen Mary Hospital, 102 Pokfulam Road, Hong Kong SAR, China.
Tel: (852) 2255 3064; Fax: (852) 2255 1345; Email: eyhchen@hku.hk
Submitted: 25 May 2010; Accepted: 9 July 2010
Abstract
Objective: To examine the spontaneous blink rate over a 3-year period and its clinical and cognitive correlates among patients with first-episode schizophrenia.
Methods: This study prospectively followed 93 patients with first-episode schizophrenia, schizophreniform and schizoaffective disorders for 3 years. Patients were longitudinally assessed for blink rate, their positive and negative symptoms, and a range of cognitive features including verbal fluency, verbal memory, visual memory, and the Wisconsin Card Sorting Test performance.
Results: When compared with a matched control group, there was a significantly higher blink rate at their 3-year follow-up but not at initial presentation. The increase in blink rate over time correlated positively with the number of relapses. It also correlated with logical memory, verbal fluency, categories completed, and perseverative errors in the Wisconsin Card Sorting Test. The increased blink rate also correlated with pre-morbid schizoid and schizotypal traits. All these correlations were statistically significant.
Conclusion: The change in the blink rate over time may reflect underlying involvement of the dopaminergic system in mediating relapse and cognitive functions.
Key words: Blinking; Cognition; Dopamine; Recurrence; Schizophrenia
摘要
目的:检视首发精神分裂症患者3年内其自发性眨眼率和有关临床和认知功能的相互关係。
方法:研究以3年时间跟进93名首发精神分裂症、类精神分裂症或分裂情感性疾患者,追踪评 估他们的眨眼率、正面和负面徵状和各种认知行为,包括口头流畅、口头记忆、视觉记忆和威 斯康卡片排序测试。
结果:与对照组比较,患者3年後的眨眼率较入院时显著上升;这跟病情复发次数显著相关,也 与逻辑记忆、口头流畅、类别完成,以及威斯康卡片排序测试的持续性错误呈相关性。眨眼率 的增加也与病前的类精神分裂人格异常和分裂性人格障碍特徵相关。以上相关性都是显著的。
结论:多巴胺系统的运作或影响病情复发和认知功能,从而改变患者的眨眼率。
关键词:眨眼、认知、多巴胺、复发、精神分裂症
Introduction
It is generally accepted that the central dopaminergic system is implicated in the pathogenesis of schizophrenia.1-3
Measures of dopamine system stability and sensitivity have been linked to proneness to psychotic episodes,4-6 tendency to relapse,7-9 and cognitive performance.10-14 Despite such considerations, direct measurement of central dopaminergic activities has relied on radiotracer neuroimaging techniques, which are complex and invasive. One of the few clinical measures that has been investigated as a measure of central dopaminergic activity is the spontaneous eye-blink rate.15-17 D1 and D2 agonists and antagonists, which can selectively enhance and block central dopaminergic activity,18-20 have been shown to increase and decrease the blink rate, respectively.21
The blink rate is generally increased in schizophrenia,15-17,22,23 but these conclusions stem from studies of chronic schizophrenic patients. It is important to investigate the blink rate of first-episode schizophrenia patients, because the frequency might change with treatment over time. For instance, it has been found that an increase in the blink rate is more consistently noted in antipsychotic- naїve than treated patients.23-25 In addition, the reduction in the blink rate following antipsychotic medication26,27 is only evident in medication-naїve patients and not in those who have been medicated.28,29 It is possible that the blockage of the dopamine receptor by antipsychotics might induce postsynaptic supersensitivity to dopamine,30 and thus result in increased blink rates and the altered responsiveness of the blink rate following antipsychotics.
Despite the recognition that dopaminergic activity plays a central role in cognitive functions, few studies have addressed the relationship between the blink rate and neurocognitive functions. Chen et al31 did not detect a link between the blink rate and neurocognitive function. In a cross-sectional study of chronic schizophrenic patients, correlations were found between the blink rate and general psychopathology and soft disinhibition.32 If the blink rate reflects central dopaminergic activities, its level may also be linked to the propensity to relapse. Lieberman et al9 used a methylphenidate stimulation test in a sample of 34 chronic schizophrenic patients, and found that the activation in the blink rate after methylphenidate administration was related to the time to relapse. Few other studies have addressed the relationship between blink rates and relapse.
Although most of the existing studies were cross- sectional,23,25,33,34 investigating changes in the blink rate longitudinally may also be important because they could enable within-subject (as opposed to between-subject) variations to be noted. Moreover, their relationship to antipsychotic medication could be evaluated. Conceivably some correlates with blink rate may be more sensitively detected by such within-subject comparisons. The few existing longitudinal studies of blink rate entailed short- term follow-up durations (4-12 weeks).26,27,35 Studies with longer follow-up might be informative.
In order to address this dearth in the literature, we set out to investigate the blink rate and its correlates in a sample of first-episode schizophrenia patients followed up for 3 years. We wished to address the relationship between any change in blink rate and neurocognitive performance as well as the propensity of patients to relapse. We hypothesised that a decrease in the blink rate might correlate with the level of neurocognitive impairment, whereas an increase relate to the tendency to relapse.
Methods
Participants
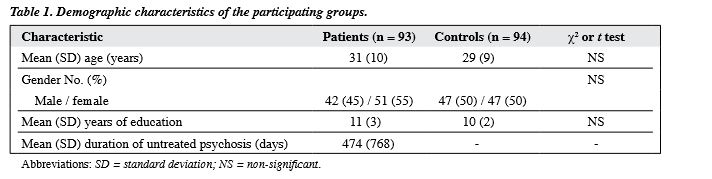
The sample consisted of patients conforming to the diagnosis of first-episode schizophrenia, schizophreniform psychosis, or schizoaffective disorder, according to the 4th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). Patients aged from 18 to 55 years were recruited from adult psychiatric services covering a defined catchment area with a population of 1.3 million in Hong Kong. Patients were excluded if they had experienced previous psychotic episodes (whether treated or not), if they had a known neurological condition or movement disorders (e.g. tics), or if there was a history of special school attendance (locally, attendance at a special school usually indicated the presence of a moderate-to-severe learning disability). All patients were treated with low- dose conventional antipsychotics (<5 mg of haloperidol or its equivalent). The study was approved by the relevant institutional review board and all of the subjects gave their written informed consent before participation. Control subjects were recruited from a healthy volunteer pool, after screening by means of a questionnaire to exclude any history of mental illness or antipsychotic medication.
The patients were assessed at the point of first contact and after the stabilisation of the first psychotic episode (a mean of 43 days after the initial assessment) when no major change in the level of symptoms or treatment was anticipated. Follow-up assessments were carried out annually for 3 years.
Blink Rate
The blink rate was assessed by an experimenter blinded to clinical ratings. Patients were asked to relax while listening to a passage of slow string music. In this state, their blink rate was monitored for 2 minutes and manually recorded with a mechanical counter. Participants were unaware that their eye-blink rate was being measured when listening to music. Upon completing the 2-minute session, they were informed that the relaxation task was over.
Cognitive Assessment
A logical memory test was administered, as described in the Wechsler Memory Scale Revised36 (adapted for Cantonese- speaking patients, Wong CW, personal communication). Verbal fluency was assessed as the number of words (repeated items and items clearly outside the category were not counted) a patient could produce in 1 minute for the category ‘animal’. Executive function was assessed with the Modified Wisconsin Card Sorting Test (MWCST).37 Perseverative errors and categories completed were calculated.
Clinical Assessment
Diagnoses were made according to the DSM-IV criteria38 based on clinical interviews, informant histories, and medical records. An inter-rater agreement for the diagnosis at the level of the main disease categories in a validation sample of 38 cases was 86%.39 Symptoms were assessed using the positive and negative symptom scale.40 The Abnormal Involuntary Movement Scale41 (AIMS) was used to measure dyskinesia. Patients were also assessed for extrapyramidal symptoms using the Simpson- Angus (SA) scale.42 Relapse was defined as a significant deterioration in terms of positive symptoms (hallucination, delusions and language disorganisation) deemed to require pharmacological intervention or hospitalisation, and was assessed by concurrent clinical assessments every 4 months. The Calgary Depression Scale for schizophrenics43 was used to assess depressive symptoms. The patients’ pre-morbid personality and adjustment were assessed by interviewing their parents using the Pre-morbid Schizoid and Schizotypal Trait scale (PSST)44 and an abridged version of the Pre- morbid Adjustment Scale45 (PAS). The duration of untreated psychosis (DUP) was evaluated using the Interview for the Retrospective Assessment of the Onset of Schizophrenia, which is a semi-structured instrument designed to capture detailed information relating to the onset and early course of psychotic symptoms.46 Patients and informants were interviewed to determine the date of first occurrences of key symptoms. The DUP was calculated as the period between the onset date of the earliest psychotic symptom and the date of initiation of systemic pharmacotherapy.
Statistical Analyses
Statistical analyses were carried out using the Statistical Package for the Social Sciences Windows version 17.0. For each patient, we first produced descriptive data of the blink rate and calculated the difference in the rate at initial presentation and the end of the 3-year follow-up. Independent sample t tests were used to compare the blink rates of drug-naïve and medicated patients, and patients receiving different types of antipsychotics (i.e. atypical vs. typical medication). Pearson corrections were applied to explore correlations between longitudinal change of the relaxation blink rate and clinical and cognitive variables. A linear regression model was used to estimate the relative contribution of different clinical and cognitive variables towards the change in blink rate. Throughout the data analysis, 2-tailed significance tests were used.
Results
In all, 153 first-episode psychosis patients and 94 controls (47 men, 47 women; mean [standard deviation (SD)] age, 29 [9] years) were initially recruited into the study. In the patient sample, 5 patients had died by the third year, and of the remaining patients, 93 (42 men and 51 women; mean [SD] age, 31 [10] years) completed the assessment at 3 years (a retention rate of 61%). The mean (SD) DUP was 474 (768) days and the mean (SD) educational level was 11 (3) years. The DSM-IV diagnoses for the sample were schizophrenia (n = 75), schizophreniform psychosis (n = 13), and schizoaffective disorder (n = 5). Forty-eight patients were first assessed in a medication-naїve state. The rest were assessed within 7 days of starting medication. Patients and controls were matched for age, gender, and years of education (Table 1).
The mean (SD) blink rate at initial presentation was 20 (18) blinks / min. At the end of the 3-year follow-up, there was a significant increase of the blink rate to 25 (21) blinks / min (t = –2.86, p = 0.01). At initial presentation, the blink rate in patients was not significantly higher than that in the controls (16 [16] blinks / min; t = 1.59, p = 0.11). However, at the 3-year follow-up, the blink rate in patients was significantly higher (t = 3.69, p < 0.01). There was no significant difference in blink rates between drug-naïve and medicated patients at initial assessment. Neither was there a difference in blink rates between those taking typical and atypical medication.
The relationship between changes in blink rate and neurocognitive performance at the end of the 3 years was explored by correlation analyses. The change in blink rate over time correlated significantly with logical memory (r = 0.25, p < 0.05), verbal fluency (r = 0.29, p < 0.01), categories completed in the MWCST (r = 0.23, p < 0.05), and the number of perseverative errors in the MWCST (r = –0.29, p < 0.01). There was no significant correlation between the change in the blink rate and visual reproduction or digit span scores.
There was a positive correlation between the change in blink rate and the total number of relapses in the 3 years (r = 0.30, p < 0.01), the AIMS score (r = 0.24, p < 0.05), and the PSST scores (r = 0.23, p = 0.03). Further analysis revealed a significant correlation with the suspiciousness item (r = 0.25, p = 0.02) and the thought item (r = 0.28, p = 0.01) in the PSST. However, there was no correlation with the PAS scores. There was a negative correlation between the blink rate and the SA score (r = –0.23, p < 0.05). There was no significant correlation between changes in blink rate and positive or negative symptoms, medication dosage, age and education level of the patient, or the DUP.
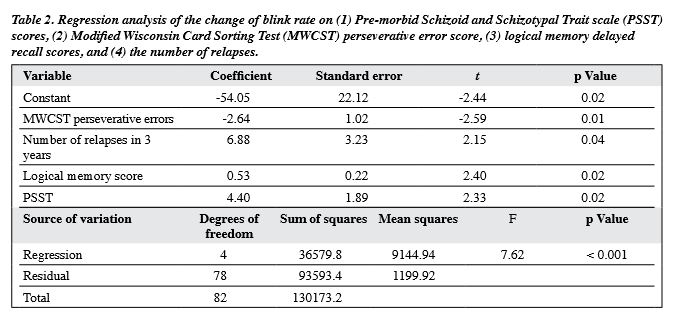
A forward stepwise linear regression analysis was carried out with the blink rate as the dependent variable, and the scores for AIMS, PSST, MWCST perseverative errors, logical memory, verbal fluency, and number of relapses all used as independent variables. The final model accounted for 24% of the total variance, and included scores for perseverative errors, logical memory, the number of relapses, and PSST as significant predictors (Table 2).
Discussion
The change in the blink rate over the first 3 years in the course of a schizophrenic illness is related to a tendency towards relapse, dyskinesia, pre-morbid schizoid and schizotypal traits, as well as to specific neurocognitive impairments.
The significant correlation between the change of the blink rate over time and the total number of relapses in 3 years was consistent with the view that activity of the dopaminergic system is implicated in relapse.7-9 This finding is also in keeping with the results of Lieberman et al8 that the activation in the blink rate following methylphenidate challenge is related to relapse. It has been suggested that, in schizophrenia, neurodevelopmental abnormalities of prefrontal dopaminergic systems might result in a state of enhanced vulnerability to sensitisation during late adolescence and early adulthood.47,48 The single-photon emission computed tomography receptor studies have also supported the notion that the development of psychosis is linked to an unstable / sensitised dopaminergic system.49-51 It seems reasonable to propose that stability of the latter system may also be related to the patient relapse.7-9 Yet, it is a limitation that other factors potentially affecting relapse (i.e. medication adherence, life events) were not studied in the current study. Prior to drawing any authoritative conclusions from this work, a follow-up study addressing these possible confounders has to be conducted. In addition, antipsychotic treatment might sensitise the dopaminergic system, causing mesolimbic postsynaptic dopamine supersensitivity.52-55 Our findings that an increased blink rate is associated with tardive dyskinesia (namely, the correlation between the AIMS score and change of blink rate) is consistent with this notion of supersensitivity of dopamine receptors.56
Our findings also confirm that a reduction of blink rate is associated with neurocognitive impairment in first- episode schizophrenia patients. The significant relationship found between the blink rate, logical memory, verbal fluency and the MWCST is consistent with the suggested role of dopaminergic functions in these cognitive domains. It has been recognised that dopaminergic activity plays a significant role in attention,57-59 learning,10,60 working memory12-14,61-64 and episodic memory.12,60 The correlation between the blink rate and performance in the MWCST can also be understood in terms of involvement with the dopaminergic system.65-68 The relationship between the blink rate and logical memory is noteworthy, as episodic verbal memory is not normally considered to be strongly under the influence of the dopamine system. However, it has been pointed out that performance in episodic memory tasks may require ‘cognitive control’, which is modulated by dopamine system activity.12 Changes in the blink rate were also related to pre-morbid schizoid and schizotypal traits. This relationship was particularly strong with the suspiciousness item and the thought item in the PSST, which suggests that these dimensions are possibly related to dopaminergic functions. The relationship between schizotypal features and dopaminergic activities has been described in previous studies.11,69,70 We found that pre-morbid schizotypal traits were associated with longitudinal changes in the blink rate following first-episode schizophrenia.
As all patients in our study received conventional antipsychotic medication, it was expected that medication may have contributed to the relationship between the blink rate and its correlates. However, we did not find a direct correlation between medication dosage, the blink rate, relapse status, neurocognitive functions, and dyskinesia. Although this lack of relationship suggests that there is no linear relationship with medication, more subtle effects of medication cannot be entirely excluded. Interestingly, there was an overall increase in the blink rate from the initial presentation to the end of the 3-year follow-up. Although studies have shown a short-term reduction of the blink rate following antipsychotic treatment in medication-naїve patients,26,27 this decrease was not detected in our medicated patients.28,29 Thus, long-term antipsychotic therapy may alter the dopamine system, possibly by modulating the sensitivity of the dopamine receptors. This was in keeping with the finding that an increase in blink rates over time correlates with dyskinesia scores.30
We found that the change in the blink rate over time following first-episode schizophrenia was related to a number of the clinical and cognitive manifestations of the disorder. These appeared to reflect the underlying involvement of different aspects of the dopaminergic system. Our study illustrates that it is important to assess changes longitudinally. Although many of the relationships are modest, the findings support the validity of considering spontaneous blink rate as a measure of dopaminergic activity. The advantage of considering the blink rate is that it is a low-cost and low-burden form of non-invasive assessment, feasibly for deployment in most clinical settings.
Acknowledgement
The study was supported by Research Grants Council of the Hong Kong Special Administrative Region, China (Grant no: 10202402.20376.21500). The authors would also like to express sincere thanks to Ms J Longenecker for editing the manuscript.
References
- Bennett MR. Monoaminergic synapses and schizophrenia: 45 years of neuroleptics. J Psychopharmacol 1998;12:289-304.
- Carlsson A, Waters N, Holm-Waters S, Tedroff J, Nilsson M, Carlsson ML. Interactions between monoamines, glutamate, and GABA in schizophrenia: new evidence. Annu Rev Pharmacol Toxicol 2001;41:237-60.
- Kasper S. Dopaminergic deficit and the role of amisulpride in the treatment of schizophrenia. Int Clin Psychopharmacol 2002;17 Suppl 4:S19-26.
- Ballmaier M, Zoli M, Leo G, Agnati LF, Spano P. Preferential alterations in the mesolimbic dopamine pathway of heterozygous reeler mice: an emerging animal-based model of schizophrenia. Eur J Neurosci 2002;15:1197-205.
- Fudge JL, Emiliano AB. The extended amygdala and the dopamine system: another piece of the dopamine puzzle. J Neuropsychiatry Clin Neurosci 2003;15:306-16.
- Kapur S, Mamo D. Half a century of antipsychotics and still a central role for dopamine D2 receptors. Prog Neuropsychopharmacol Biol Psychiatry 2003;27:1081-90.
- Lieberman JA. Prediction of outcome in first-episode schizophrenia. J Clin Psychiatry 1993;54 Suppl:S13-7.
- Lieberman JA, Alvir J, Geisler S, Ramos-Lorenzi J, Woerner M, Novacenko H, et al. Methylphenidate response, psychopathology and tardive dyskinesia as predictors of relapse in schizophrenia. Neuropsychopharmacology 1994;11:107-18.
- Lieberman JA, Kane JM, Sarantakos S, Gadaleta D, Woerner M, Alvir J, et al. Prediction of relapse in schizophrenia. Arch Gen Psychiatry 1987;44:597-603.
- Sigala S, Missale C, Spano P. Opposite effects of dopamine D2 and D3 receptors on learning and memory in the rat. Eur J Pharmacol 1997;336:107-12.
- Siever LJ, Koenigsberg HW, Harvey P, Mitropoulou V, Laruelle M, Abi-Dargham A, et al. Cognitive and brain function in schizotypal personality disorder. Schizophr Res 2002;54:157-67.
- Dujardin K, Laurent B. Dysfunction of the human memory systems: role of the dopaminergic transmission. Curr Opin Neurol 2003;16 Suppl 2:S11-6.
- Marié RM, Defer GL. Working memory and dopamine: clinical and experimental clues. Curr Opin Neurol 2003;16 Suppl 2:S29-35.
- Zhang K, Grady CJ, Tsapakis EM, Andersen SL, Tarazi FI, Baldessarini RJ. Regulation of working memory by dopamine D4 receptor in rats. Neuropsychopharmacology 2004;29:1648-55.
- Karson CN. Spontaneous eye-blink rates and dopaminergic systems. Brain 1983;106:643-53.
- Stevens JR. Eye blink and schizophrenia: psychosis or tardive dyskinesia? Am J Psychiatry 1978;135:223-6.
- Sandyk R. The significance of eye blink rate in parkinsonism: a hypothesis. Int J Neurosci 1990;51:99-103.
- Blin O, Masson G, Azulay JP, Fondarai J, Serratrice G. Apomorphine- induced blinking and yawning in healthy volunteers. Br J Clin Pharmacol 1990;30:769-73.
- Lawrence MS, Redmond DE Jr, Elsworth JD, Taylor JR, Roth RH. The D1 receptor antagonist, SCH 23390, induces signs of parkinsonism in African green monkeys. Life Sci 1991;49:PL229-34.
- Hu P, Chen L, Zhang HQ, Li JR, Liang H. Single photon emission computer tomography of dopamine transporters in monkeys and humans with 99mTc-TRODAT-1. Chin Med J (Engl) 2004;117:1056- 9.
- Taylor JR, Elsworth JD, Lawrence MS, Sladek JR Jr, Roth RH, Redmond DE Jr. Spontaneous blink rates correlate with dopamine levels in the caudate nucleus of MPTP-treated monkeys. Exp Neurol 1999;158:214-20.
- Helms PM, Godwin CD. Abnormalities of blink rate in psychoses: a preliminary report. Biol Psychiatry 1985;20:103-6.
- Mackert A, Woyth C, Flechtner KM, Volz HP. Increased blink rate in drug-naive acute schizophrenic patients. Biol Psychiatry 1990;27:1197-202.
- Colpaert FC, Degryse AD, Van Craenendonck HV. Effects of an alpha 2 antagonist in a 20-year-old Java monkey with MPTP-induced parkinsonian signs. Brain Res Bull 1991;26:627-31.
- Mackintosh JH, Kumar R, Kitamura T. Blink rate in psychiatric illness. Br J Psychiatry 1983;143:55-7.
- Karson CN, Bigelow LB, Kleinman JE, Weinberger DR, Wyatt RJ. Haloperidol-induced changes in blink rates correlate with changes in BPRS score. Br J Psychiatry 1982;140:503-7.
- Kleinman JE, Karson CN, Weinberger DR, Freed WJ, Berman KF, Wyatt RJ. Eye-blinking and cerebral ventricular size in chronic schizophrenic patients. Am J Psychiatry 1984;141:1430-2.
- Mackert A, Woyth C, Flechtner M, Frick K. Increased blink rate in acute and remitted schizophrenics. Pharmacopsychiatry 1988;21:334- 5.
- Mackert A, Flechtner KM, Woyth C, Frick K. Increased blink rates in schizophrenics. Influences of neuroleptics and psychopathology. Schizophr Res 1991;4:41-7.
- Mackay AV, Iversen LL, Rossor M, Spokes E, Bird E, Arregui A, et al. Increased brain dopamine and dopamine receptors in schizophrenia. Arch Gen Psychiatry 1982;39:991-7.
- Chen EY, Lam LC, Chen RY, Nguyen DG. Blink rate, neurocognitive impairments, and symptoms in schizophrenia. Biol Psychiatry 1996;40:597-603.
- Chan RC, Chen EY. Blink rate does matter: a study of blink rate, sustained attention, and neurological signs in schizophrenia. J Nerv Ment Dis 2004;192:781-3.
- Jacobsen LK, Hommer DW, Hong WL, Castellanos FX, Frazier JA, Giedd JN, et al. Blink rate in childhood-onset schizophrenia: comparison with normal and attention-deficit hyperactivity disorder controls. Biol Psychiatry 1996;40:1222-9.
- Swarztrauber K, Fujikawa DG. An electroencephalographic study comparing maximum blink rates in schizophrenic and nonschizophrenic psychiatric patients and nonpsychiatric control subjects. Biol Psychiatry 1998;43:282-7.
- Adamson TA. Changes in blink rates of Nigerian schizophrenics treated with chlorpromazine. West Afr J Med 1995;14:194-7.
- The Wechsler Adult Intelligence Scale — revised (Cantonese version). Hong Kong: Hong Kong Psychological Society; 1989.
- Heaton RK. Wisconsin Card Sorting Test manual. Odessa, Florida: Psychological Assessment Resources, Inc.; 1981.
- American Psychiatric Association. DSM-IV: Diagnostic and Statistical Manual of Mental Disorders, 4th edition, revised. Washington, DC: American Psychiatric Association; 1994.
- Chen EY, Dunn EL, Miao MY, Yeung WS, Wong CK, Chan WF, et al. The impact of family experience on the duration of untreated psychosis (DUP) in Hong Kong. Soc Psychiatry Psychiatr Epidemiol 2005; 40:350-6.
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 1987;13:261-76.
- Guy W. Abnormal involuntary movement scale (AIMS). In: Guy W, editor. ECDEU assessment manual for psychopharmacology. Revised ed. Dept of Health, Education and Welfare, National Institute of Mental Health; 1976: 534-7.
- Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl 1970;212:11-9.
- Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res 1990;3:247-51.
- Foerster A, Lewis S, Owen M, Murray R. Pre-morbid adjustment and personality in psychosis. Effects of sex and diagnosis. Br J Psychiatry 1991;158:171-6.
- Cannon-Spoor HE, Potkin SG, Wyatt RJ. Measurement of premorbid adjustment in chronic schizophrenia. Schizophr Bull 1982;8:470-84.
- Häfner H, Riecher-Rössler A, Hambrecht M, Maurer K, Meissner S, Schmidtke A, et al. IRAOS: an instrument for the assessment of onset and early course of schizophrenia. Schizophr Res 1992;6:209-23.
- Laruelle M. The role of endogenous sensitization in the pathophysiology of schizophrenia: implications from recent brain imaging studies. Brain Res Brain Res Rev 2000;31:371-84.
- Akiyama K, Kanzaki A, Tsuchida K, Ujike H. Methamphetamine- induced behavioral sensitization and its implications for relapse of schizophrenia. Schizophr Res 1994:12:251-7.
- Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci U S A 2000;97:8104-9.
- Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D’Souza CD, Erdos J, et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci U S A 1996;93:9235-40.
- Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, et al. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci U S A 1997;94:2569-74.
- Kirkpatrick B, Alphs L, Buchanan RW. The concept of supersensitivity psychosis. J Nerv Ment Dis 1992;180:265-70.
- Hunt JI, Singh H, Simpson GM. Neuroleptic-induced supersensitivity psychosis: retrospective study of schizophrenic inpatients. J Clin Psychiatry 1988;49:258-61.
- Chouinard G, Jones BD. Neuroleptic-induced supersensitivity psychosis: clinical and pharmacologic characteristics. Am J Psychiatry 1980;137:16-21.
- Davis KL, Rosenberg GS. Is there a limbic system equivalent of tardive dyskinesia? Biol Psychiatry 1979;14:699-703.
- Burt DR, Creese I, Snyder SH. Antischizophrenic drugs: chronic treatment elevates dopamine receptor binding in brain. Science 1977;196:326-8.
- Fried I, Wilson CL, Morrow JW, Cameron KA, Behnke ED, Ackerson LC, et al. Increased dopamine release in the human amygdala during performance of cognitive tasks. Nat Neurosci 2001;4:201-6.
- Goldman-Rakic PS. The cortical dopamine system: role in memory and cognition. Adv Pharmacol 1998;42:707-11.
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol 1998;80:1-27.
- Bäckman L, Farde L. Dopamine and cognitive functioning: brain imaging findings in Huntington’s disease and normal aging. Scand J Psychol 2001;42:287-96.
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, et al. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci U S A 2003;100:6186-91.
- Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science 1991;251:947-50.
- Sawaguchi T, Goldman-Rakic PS. The role of D1-dopamine receptor in working memory: local injections of dopamine antagonists into the prefrontal cortex of rhesus monkeys performing an oculomotor delayed-response task. J Neurophysiol 1994;71:515-28.
- Didriksen M. Effects of antipsychotics on cognitive behaviour in rats using the delayed non-match to position paradigm. Eur J Pharmacol 1995;281:241-50.
- Weinberger DR, Berman KF, Illowsky BP. Physiological dysfunction of dorsolateral prefrontal cortex in schizophrenia. III. A new cohort and evidence for a monoaminergic mechanism. Arch Gen Psychiatry 1988;45:609-15.
- Roberts AC, De Salvia MA, Wilkinson LS, Collins P, Muir JL, Everitt BJ, et al. 6-Hydroxydopamine lesions of the prefrontal cortex in monkeys enhance performance on an analog of the Wisconsin Card Sort Test: possible interactions with subcortical dopamine. J Neurosci 1994;14:2531-44.
- Kahn RS, Harvey PD, Davidson M, Keefe RS, Apter S, Neale JM, et al. Neuropsychological correlates of central monoamine function in chronic schizophrenia: relationship between CSF metabolites and cognitive function. Schizophr Res 1994;11:217-24.
- Amos A. A computational model of information processing in the frontal cortex and basal ganglia. J Cogn Neurosci 2000;12:505-19.
- Siever LJ, Amin F, Coccaro EF, Bernstein D, Kavoussi RJ, Kalus O, et al. Plasma homovanillic acid in schizotypal personality disorder. Am J Psychiatry 1991;148:1246-8.
- Abi-Dargham A, Kegeles LS, Zea-Ponce Y, Mawlawi O, Martinez D, Mitropoulou V, et al. Striatal amphetamine-induced dopamine release in patients with schizotypal personality disorder studied with single photon emission computed tomography and [123I]iodobenzamide. Biol Psychiatry 2004;55:1001-6.