East Asian Arch Psychiatry 2012;22:25-30
ORIGINAL ARTICLE
Cognitive and Functional Impairment in Chinese Elderly with Late-onset Depression
华裔老年晚发性抑鬱症患者的认知和功能缺损研究
谭焕芝、林翠华
Dr Cindy Woon-Chi Tam, FHKCPsych, FHKAM (Psychiatry), Department of Psychiatry, North District Hospital, Hong Kong SAR, China.
Prof Linda Chiu-Wa Lam, FHKCPsych, FHKAM (Psychiatry), Department of Psychiatry, The Chinese University of Hong Kong, Hong Kong SAR, China.
Address for correspondence: Dr Cindy Woon-Chi Tam, Department of Psychiatry, Psychiatric Outpatient Department, North District Hospital,
9 Po Kin Road, Sheung Shui, New Territories, Hong Kong SAR, China. Tel: (852) 2683 7620; Fax: (852) 2683 7616;
Email: tamwoonchi@hotmail.com
Submitted: 19 September 2011; Accepted: 1 December 2011
Abstract
Objectives: To investigate cognitive and functional impairment in Chinese elderly subjects with late- onset depression.
Methods: Subjects with late-onset depression and who were clinically non-demented were recruited. Their cognitive and functional scores were compared with those of cognitively normal elderly controls and elderly persons with mild cognitive impairment. Functional ability was assessed by the Disability Assessment for Dementia score. Various cognitive domains were assessed including global cognitive function, delayed episodic memory, working memory, and categorical verbal fluency test.
Results: A total of 105 depressed subjects and 324 non-depressed controls (149 normal elderly controls and 175 with mild cognitive impairment) were recruited. The depression group had significantly poorer performance in all cognitive assessments compared to the normal elderly control group. The depression group had a similar cognitive profile to those with mild cognitive impairment, except that its subjects had slightly better performance in the Categorical Verbal Fluency Test, delayed recall testing, and the Chinese version of the Alzheimer’s Disease Assessment Scale–Cognitive subscale test. Depressed subjects had significantly lower functional scores in instrumental activities of daily living than the non-depressed, normal elderly controls, and those with mild cognitive impairment.
Conclusions: Our results demonstrate that Chinese elderly with late-onset depression had cognitive impairments in multiple domains similar to those encountered in the age- and sex-matched non-depressed controls with mild cognitive impairment. However, their functional performance was significantly poorer than that in these controls. This study provided extensive characterisation of the range and depth of cognitive and functional impairments in elderly patients with late-onset depression.
Key words: Age of onset; Cognition disorders; Depression
摘要
目的:检视华裔老年晚发性抑鬱症患者的认知和功能缺损。
方法:纳入晚发性抑鬱症患者和认知正常的患者,并将两者的认知和功能得分与轻度认知缺损患者进行比较。研究参照脑退化症失能量表评估患者功能,并观察整体认知功能、延宕事件记忆、工作记忆和类别性语言流畅度测试等各种认知域。
结果:共纳入105例抑鬱症患者和324例非抑鬱症对照组(包括149例正常对照老人和175例轻度认知缺损患者)。与正常对照老人比较,抑鬱症患者在所有认知评估的表现皆明显较差。虽然抑鬱症患者与轻度认知缺损患者的认知模式相近,但前者於类别性语言流畅度测试、延宕回忆测试,以及中文版阿兹海默氏病量表 —— 认知子量表的表现均略佳。不过,抑鬱症患者在工具性日常生活活动量表的功能得分则明显不及正常对照老人和轻度认知缺损患者。
结论:研究结果显示,虽然华裔老年晚发性抑鬱症患者在多个认知域均呈现认知缺损,情况与年龄和性别匹配的轻度认知缺损对照患者类近,但前者的功能表现明显不及对照群。本文并对老年晚发性抑鬱症患者其认知和功能缺损作广泛探讨。
>关键词:发病年龄、认知障碍、抑鬱症
Introduction
Given that the personal and public burdens of both depression and cognitive impairment are likely to increase along with the ageing population, it is important for mental health professionals to better understand the characteristics of cognitive impairment in depression, as well as how to detect and treat it.
There is increasing recognition that cognitive impairment occurs in geriatric depression, and that its presentation is heterogeneous. Most research has found that depressed individuals tend to have worse performance relative to non-depressed comparison groups on a number of neuropsychological measures. The most consistent deficits occur in the areas of processing speed1-3; effortful tasks involving selective attention, response inhibition, and performance monitoring (i.e. executive functions)4,5; and the acquisition and retrieval of new information (i.e. episodic memory).5 Neurocognitive deficits involving executive dysfunction are common when the episode of depression occurs in late life. Some research suggests that memory deficits may be more focally affected among older individuals with a history of recurrent depression beginning earlier in life.6
Studies involving multiple cognitive domains found that 33 to 50% of depressed individuals have clinical levels of cognitive impairment.7,8 The presence of depression (particularly in late-onset cases) should raise the possibility of screening for cognitive impairment as part of a long-term approach to care.
Depression has considerable influence on functional impairment and disability. The relationship between disability and depression is complex and probably bidirectional. In a systematic review of variables predicting functional decline in community-dwelling older adults, depression was one of the key risk factors.9 Impairment in activities of daily living (ADL) is likely an underappreciated feature of depression and one that has important effects on dementia outcomes. Functional impairment may also be a marker for adverse outcomes. Among depressed individuals, self-reported impairments in instrumental activities of daily living (IADL) are associated with more pervasive cognitive impairment and persistence of impairment after depression remits.10,11
Thus, evidence of functional impairment among individuals with depression may be a warning sign of individuals who are at risk for cognitive decline and that this risk may be lowered by effective identification and treatment.
In Hong Kong, there is a paucity of studies on the cognitive and functional profiles of subjects with late-onset depression. Our study aimed to investigate cognitive and functional impairment in Chinese elderly subjects with late-onset depression. We hypothesised that such elderly had impairment in cognition and IADL compared with non-depressed age- and education-matched controls. The corresponding findings could provide more information to characterise the clinical presentation of late-onset depressive syndromes.
Methods
Sample
Depressed Subjects
Patients aged ≥ 60 years who fulfilled the DSM-IV criteria12 for major or minor depression were recruited from psychiatric outpatient clinics and the inpatient psychiatric unit. The onset of the first depressive episode was aged ≥ 50 years.
Each depressed subject was evaluated by a qualified psychiatrist to establish eligibility for inclusion in the study and a clinical diagnosis, and to assess clinical staging based on the Clinical Dementia Rating (CDR) scale.13 Subjects with a global CDR score of 0 or 0.5 were recruited.
Controls
The control subjects were recruited from a population- based epidemiology study of cognitive impairment in elderly conducted from October 2005 to July 2006.14 The subjects had been assessed by an experienced psychiatrist and were neither clinically demented nor depressed. All of the subjects were ≥ 60 years with no history or current depression. Control subjects with CDR of 0 were classified as cognitively normal controls (NC). Control subjects with CDR of 0.5 and delayed recall test score of > 1.5 standard deviation (SD) below the mean of the NC were classified as having mild cognitive impairment (MCI). All controls were age- and education-matched with the depressed subjects.
We excluded subjects and controls with any prior history of degenerative neurological disorder, dementia, cortical strokes, severe or unstable physical illness, and prior or current substance / alcohol abuse. Subjects that had electroconvulsive therapy in the past 3 months were also excluded. The psychiatrists explained the procedure and obtained informed consent from the participants or their caregivers. The entire study was approved by the Joint CUHK-NTEC Clinical Research Ethics Committee of the Chinese University of Hong Kong.
Assessments
All subjects underwent a comprehensive psychiatric, cognitive, and functional assessment. Depression was diagnosed according to the DSM-IV criteria, and symptom severity was rated using the Montgomery-Asberg Depression Rating Scale (MADRS)15 and the Hamilton Depression Rating Scale (HDRS).16
Cognitive Tests
Global cognitive assessment was estimated using the Cantonese version of the Mini-Mental State Examination17 and the Chinese version of the Alzheimer’s Disease Assessment Scale–Cognitive subscale (ADAS-Cog).18 To test for episodic memory, subjects were also examined using a 10-minute delayed recall of a word list from the ADAS-Cog. Digit span and visual span tests were carried out to test attention and working memory. The Category Verbal Fluency Test (CVFT) was performed as a test of executive function. In the CVFT, subjects were asked to generate exemplars in the categories of animals, fruit, and vegetables within 1 minute. Combined scores were then computed.19
Disability Assessment for Dementia Scale
The Disability Assessment for Dementia (DAD) scale is a validated measure of ADL designed specifically for use in patients with dementia.16 The Chinese version of the DAD scale has been validated in Chinese subjects.20 Each test item is considered by the cognitive processing involved as being an initiation item, a planning and organisation item, or a performance item. Of the 47 items, 14 are assigned as initiation items, 15 as planning and organisation items, and 18 as performance items. The DAD score is expressed as a percentage, with higher scores indicating better functioning. Apart from the total DAD score, the scores for basic and IADL domains, the initiation, planning and organisation, and performance scales can also be analysed.
Statistical Analyses
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS 17.0; SPSS Inc., Chicago [IL], US). The demographic data, cognitive scores and functional scores between groups were analysed using analysis of variance with Bonferroni correction. The significance level was set at p < 0.05. The Chi-square statistics was used to compare frequencies of the categorical demographic data. Z scores of the cognitive and functional scores were calculated with reference to the mean scores of the NC group.
Results
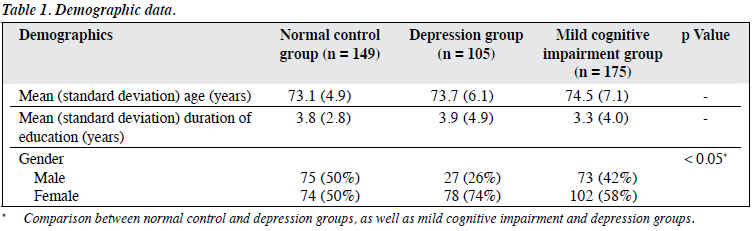
The whole sample consisted of 429 subjects; 105 subjects belonged to the depression group and 324 to the non- depressed controls. There were 149 subjects in NC group and 175 in MCI group. In all, there were 175 male and 254 female subjects.
The demographic characteristics of the subjects are summarised in Table 1. The depression and NC groups were matched in terms of age and education level. The mean (± SD) age was 74 ± 6 years and the mean (± SD) duration of education was 3.6 ± 3.9 years. The depression group had a significantly higher female-to-male ratio. In the depression group, the respective mean (± SD) MADRS and HDRS scores were 23 ± 9 and 18 ± 8. The mean depressive scores reflected that they had mild-to-moderate depression.
Cognitive Profiles
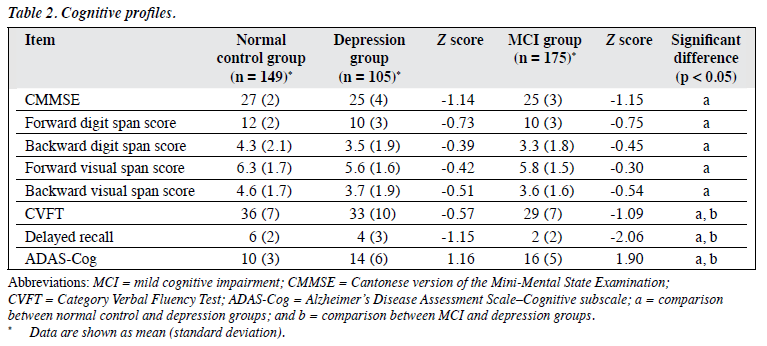
The depression group showed significant cognitive impairment compared to the NC group in all cognitive tests including global cognitive function, delayed recall memory, working memory, and CVFT. The depression group had more prominent impairment in episodic memory than for other cognitive domains, as reflected in the Z scores (Fig 1). The depression group had significantly better performance than the MCI group in the ADAS-Cog, delayed recall test, and CVFT. The MCI group had more prominent impairment in episodic memory and verbal fluency compared to the NC group. Of 105 depressed subjects, 61 (58%) had a global CDR of 0.5, 51 (47%) had a delayed recall test score of > 1.5 SD below the mean of the NC group. Details of the cognitive profiles are shown in Table 2.
Functional Performance
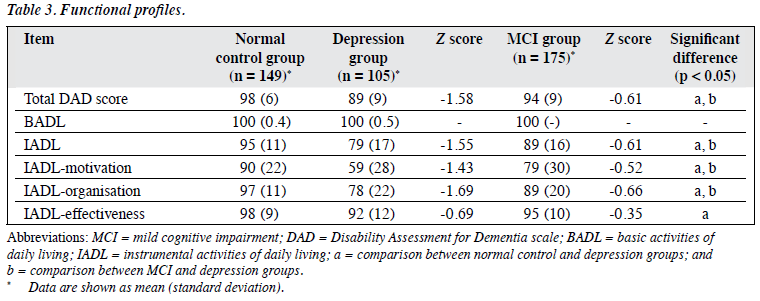
The depression group had poorer functional performance than the NC and MCI groups in terms of IADL. As reflected by the Z scores, the impairment was more prominent for the motivation, as well as organisation and planning subscores (Fig 2). There were no significant differences in basic ADL between depression and NC groups. Details are also shown in Table 3.
Discussion
This study provided a characterisation of the cognitive and functional profiles in Chinese elderly with late- onset depression. Depressed subjects showed cognitive impairments affecting episodic memory, working memory, executive functions and global functions compared to the age- and education-matched NC group. Consistent with the literature, impairment was found to affect multiple cognitive domains, including episodic memory, executive functioning, and processing speed.21
The most consistent cognitive deficits observed in depressed patients, who do not meet criteria for early dementia, are slowed processing speed, deficits in executive function and memory.5,22-24 Executive function dysfunction and / or slowed processing speed (both putative indices of frontostriatal dysfunction) mediate other cognitive weaknesses in geriatric depression, including poor visual spatial skills and episodic memory.2,23,25 A recent study found that the effect of slowed processing speed on executive function deficits was greater than that on memory deficits, though its effect was not sufficient to explain deficits in other domains that exist in parallel.21 The cognitive deficits in geriatric depression may stem from a diversity of structural brain changes. Late-onset depression is associated with pathology in subcortical and deep white matter structures and more frequently occurs with cognitive impairment, typically with prominent features of executive dysfunction that reflect underlying disruption of frontostriatal circuits.26
It has been suggested that compared to other cognitive domains, measures of executive function may have good prognostic value with regard to responsiveness to treatment, whereas measures of global cognitive function and memory identify patients ‘at risk’ of dementia.5
Our results suggest that it is difficult to differentiate between control subjects with MCI and depressed subjects on the basis of their neuropsychological profiles. Zihl et al27 also reported similar neuropsychological profiles for attention, memory, and executive functions in MCI and in depression.
Although the depressed subjects had less severe cognitive impairment than the age- and education-matched community controls with MCI, they showed more marked functional impairment. This might imply that late- onset depression is associated with additional functional impairment, independent of the cognitive impairment in older persons with MCI. Functional disability may result from depressive symptoms such as fatigue or apathy, which are reflected in their lower motivation, planning, and organisational scores. Lack of interest and motivation, depressive mood, compounded by behavioural abnormalities resulting from executive dysfunction, may well account for functional disability in elderly subjects with late-onset depression.
The strengths of the present study are: (1) recourse to a heterogeneous geriatric patient group to maximise the generalisability of findings; and (2) inclusion of a large sample of elderly control subjects to account for age-related cognitive change. Regarding limitations, we have difficulty performing a comprehensive neuropsychological battery to assess all the community control subjects, particularly the executive tests and the tests for information processing speed. Moreover, our control sample had relatively low education levels and had difficulty completing the more complex executive tests. Any future study should entail more detailed tests into attention and information processing speed, so as to delineate age-related and mood-related cognitive dysfunction.
In conclusion, late-onset depression affects mood, cognition and functional ability in the elderly. Depression with cognitive deficits showed broad-based cognitive impairment, similar to that in community elderly with multi-domain MCI. However, late-onset depression was associated with impairment in initiation, organisation and planning of IADL, which was out of proportion to the cognitive deficit.
References
- Nebes RD, Butters MA, Mulsant BH, Pollock BG, Zmuda MD, Houck PR, et al. Decreased working memory and processing speed mediate cognitive impairment in geriatric depression. Psychol Med 2000;30:679-91.
- Sheline YI, Barch DM, Garcia K, Gersing K, Pieper C, Welsh-Bohmer K, et al. Cognitive function in late life depression: relationships to depression severity, cerebrovascular risk factors and processing speed. Biol Psychiatry 2006;60:58-65.
- Boone KB, Lesser IM, Miller BL, Wohl M, Berman N, Lee A, et al. Cognitive functioning in older depressed outpatients: relationship of presence and severity of depression to neuropsychological test scores. Neuropsychology 1995;9:390-8.
- Beats BC, Sahakian BJ, Levy R. Cognitive performance in tests sensitive to frontal lobe dysfunction in the elderly depressed. Psychol Med 1996;26:591-603.
- Lockwood KA, Alexopoulos GS, van Gorp WG. Executive dysfunction in geriatric depression. Am J Psychiatry 2002;159:1119-26.
- Rapp MA, Dahlman K, Sano M, Grossman HT, Haroutunian V, Gorman JM. Neuropsychological differences between late-onset and recurrent geriatric major depression. Am J Psychiatry 2005;162:691-8.
- Butters MA, Whyte EM, Nebes RD, Begley AE, Dew MA, Mulsant BH, et al. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry 2004;61:587- 95.
- Reischies FM, Neu P. Comorbidity of mild cognitive disorder and depression — a neuropsychological analysis. Eur Arch Psychiatry Clin Neurosci 2000;50:186-93.
- Stuck AE, Walthert JM, Nikolaus T, Büla CJ, Hohmann C, Beck JC. Risk factors for functional status decline in community-living elderly people: a systematic literature review. Soc Sci Med 1999;48:445-69.
- Steffens DC, Hays JC, Krishnan KR. Disability in geriatric depression. Am J Geriatr Psychiatry 1999;7:34-40.
- 1 Lee JS, Potter GG, Wagner HR, Welsh-Bohmer KA, Steffens DC. Persistent mild cognitive impairment in geriatric depression. Int Psychogeriatr 2007;19:125-35.
- American Psychiatric Association Committee on Nomenclature and Statistics. Diagnostic and statistical manual of mental disorders (DSM- IV), 4th ed. Washington, D.C.: American Psychiatric Association; 1994.
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry 1982;140:556-72.
- Lam LC, Tam CW, Lui VW, Chan WC, Chan SS, Wong S, et al. Prevalence of very mild and mild dementia in community-dwelling older Chinese people in Hong Kong. Int Psychogeriatr 2008;20:135- 48.
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979;134:382-9.
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56-62.
- Chiu HF, Lee HC, Chung WS, Kwong PK. Reliability and validity of the Cantonese version of the Mini-Mental State Examination — a preliminary study. Journal of Hong Kong College of Psychiatrists 1994;2:25-8.
- Chu LW, Chiu KC, Hui SL, Yu GK, Tsui WJ, Lee PW. The reliability and validity of the Alzheimer’s Disease Assessment Scale Cognitive Subscale (ADAS-Cog) among the elderly Chinese in Hong Kong. Ann Acad Med Singapore 2000;29:474-85.
- Lam LC, Ho P, Lui VW, Tam CW. Reduced semantic fluency as an additional screening tool for subjects with questionable dementia. Dement Geriatr Cogn Disord 2006;22:159-64.
- Mok CC, Siu AM, Chan WC, Yeung KM, Pan PC, Li SW. Functional disabilities profile of Chinese elderly people with Alzheimer’s disease — a validation study on the Chinese version of the disability assessment for dementia. Dement Geriatr Cogn Disord 2005;20:112-9.
- Thomas AJ, O’Brien JT. Depression and cognition in older adults. Curr Opin Psychiatry 2008;21:8-13.
- Lockwood KA, Alexopoulos GS, Kakuma T, van Gorp WG. Subtypes of cognitive impairment in depressed older adults. Am J Geriatr Psychiatry 2000;8:201-8.
- Butters MA, Bhalla RK, Mulsant BH, Mazumdar S, Houck PR, Begley AE, et al. Executive functioning, illness course, and relapse / recurrence in continuation and maintenance treatment of late-life depression: is there a relationship? Am J Geriatr Psychiatry 2004;12:387-94.
- Köhler S, Thomas AJ, Barnett NA, O’Brien JT. The pattern and course of cognitive impairment in late-life depression. Psychol Med 2010;40:591-602.
- Elderkin-Thompson V, Kumar A, Mintz J, Boone K, Bahng E, Lavretsky H. Executive dysfunction and visuospatial ability among depressed elders in a community setting. Arch Clin Neuropsychol 2004;19:597-611.
- Alexopoulos GS, Kiosses DN, Klimstra S, Kalayam B, Bruce ML. Clinical presentation of the “depressive-executive dysfunction syndrome” of late life. Am J Geriatr Psychiatry 2002;10:98-106.
- Zihl J, Reppermund S, Thum S, Unger K. Neuropsychological profiles in MCI and in depression: differential cognitive dysfunction patterns or similar final common pathway disorder? J Psychiatr Res 2010;44:647-54.