East Asian Arch Psychiatry 2013;23:114-119
THEME PAPER
Dr Nan Mu, MD, Division of Geriatric Psychiatry, Guangzhou Brain Hospital, Guangzhou, PR China; The First Clinical Medical School, Jinan University, Guangzhou, PR China.
Dr Shi-Chao Xu, Division of Geriatric Psychiatry, Guangzhou Brain Hospital, Guangzhou, PR China.
Dr Qing Chang, Division of Geriatric Psychiatry, Guangzhou Brain Hospital, Guangzhou, PR China.
Dr Dong-Ping Rao, Division of Geriatric Psychiatry, Guangzhou Brain Hospital, Guangzhou, PR China.
Dr Jian-Ping Chen, Division of Geriatric Psychiatry, Guangzhou Brain Hospital, Guangzhou, PR China.
Prof. Cui Ma, Division of Geriatric Psychiatry, Guangzhou Brain Hospital, Guangzhou, PR China.
Address for correspondence: Dr Nan Mu, Division of Geriatric Psychiatry, Guangzhou Brain Hospital, 36 Mingxin Road, Liwan District, Guangzhou 510370, China.
Tel: (86-20) 8126 8082; Fax: (86-20) 8189 1391; Email: gzmunan@hotmail.com
Submitted: 19 March 2013; Accepted: 10 June 2013
Abstract
Objective: To investigate the characteristics of blood lipids, insulin metabolism, and paraoxonase-2-311 (PON2-311) polymorphism among patients with Alzheimer’s disease with different types of dementia.
Methods: A total of 84 patients with Alzheimer’s disease, according to the National Institute of Neurological and Communicative Disorders and Stroke, and the Alzheimer’s Disease and Related Disorders Association clinical criteria for ‘possible Alzheimer’s disease’, and with no family history of the condition, were enrolled. They were then categorised into 3 groups (senile dementia, presenile dementia, and mixed dementia) according to the diagnostic criteria of the Chinese Classification of Mental Disorders, third edition. Polymerase chain reaction–restriction fragment length polymorphism analysis was used to determine the presence of PON2-311 polymorphism. Serum cholesterol, triglycerides, high- density lipoprotein, low-density lipoprotein, and fasting blood sugar were measured. Fasting plasma insulin was measured using chemiluminescence. The basal-state method was used to assess insulin resistance expressed as insulin sensitivity index. The cognitive rating scale of the Mini-Mental State Examination, Activities of Daily Living scale, and Hachinski Ischemic Scale were used to establish the clinical features and severity of cognitive impairment. Differences in PON2-311C/S polymorphism, serum insulin, blood glucose, blood lipids, and neuropsychological score were analysed.
Results: The serum triglyceride and cholesterol levels of the presenile dementia group were significantly higher than those of the senile and mixed dementia groups (p < 0.01). The high-density lipoprotein level of the senile dementia group was significantly higher than that of the mixed dementia group (p < 0.05). The serum insulin level of the presenile dementia group was significantly higher than that of the senile (p < 0.05) and mixed dementia groups (p < 0.01). There were no significant differences in distribution of the PON2-311 genotypes C/C, C/S, and S/S between the senile and mixed dementia groups, and no significant differences in C-allele and S-allele frequency between the 2 groups.
Conclusions: The differences in serum triglycerides, cholesterol, high-density lipoprotein, and insulin levels between Alzheimer patients with senile, presenile, and mixed dementia found in this study suggest that patients with presenile dementia should monitor their lipid and insulin metabolism. No significant differences were found for PON2-311 genotypes or allele frequencies in patients with dementia due to Alzheimer’s disease.
Key words: Alzheimer disease; Aryldialkylphosphatase; Insulin; Lipids/blood; Polymorphism, genetic
Introduction
Alzheimer’s disease (AD), one of the most common types of senile dementia, is a degenerative disease of the central nervous system. Alzheimer’s disease has become a major public health and social problem in both developed and developing countries. According to the diagnostic criteria of the Chinese Classification of Mental Disorders, third edition (CCMD-3)1 — a national classification and diagnostic system widely used by psychiatrists in China — AD dementia is divided into 3 subtypes: senile dementia, presenile dementia, and mixed dementia. The onset of AD may result from a combination of genetic predisposition and environmental factors. For sporadic AD, the apolipoprotein E (APOE) ε4 allele is the only currently recognised susceptibility gene for this form of AD.2 However, APOE ε4 accounts for less than 50% of the AD genetic variation, suggesting that there are other genetic factors involved in the onset of AD. The paraoxonase (PON) enzyme is a calcium-dependent enzyme associated with high-density lipoprotein (HDL). The PON gene family comprises 3 family members, PON1, PON2, and PON3, located on the long arm of chromosome 7.3 In a study performed in a Chinese population, Shi et al4 found that there was a significant increase in the presence of the PON2-311C allele in Han Chinese patients with AD. Through correlational studies of APOE ε4, the study also showed that 311C is a risk factor independent of APOE ε4. Furthermore, there have been several studies in China showing a relationship between onset of AD and lipids and insulin. However, as for PON2-311 polymorphism, the findings have been inconsistent.3-8
This study was therefore performed among patients with sporadic AD to investigate differences in the characteristics of PON2-311 polymorphism, blood lipids, blood glucose, serum insulin levels, and insulin sensitivity among patients with different subtypes of AD diagnosed according to the CCMD-3.
Methods
Participants
Between March 2008 and December 2010, a total of 84 AD patients from the geriatric psychiatric inpatient unit and outpatient clinic of Guangzhou Brain Hospital, Guangzhou, China were enrolled into the study. All patients met the diagnostic criteria of the National Institute of Neurological and Communicative Disorders and Stroke, and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) diagnostic criteria of ‘possible AD dementia’ with no known family history. The participants underwent computed tomography, magnetic resonance imaging, blood biochemical analysis, syphilis serology, and thyroid function tests to exclude other systemic diseases or memory impairment resulting from organic encephalopathy. Patients were classified into 3 groups of senile dementia (n = 49), presenile dementia (n = 7), and mixed dementia(n = 28) according to the diagnostic criteria of the CCMD-3.
The diagnoses were made jointly by 2 psychiatrists, one of whom was a deputy director responsible for reviewing the diagnoses and evaluations. All patients and their families gave informed consent to participate in the study.
Blood Biochemical Detection
A 5-mL sample of fasting venous blood was collected from each patient. A fully automated Architect c8000 chemistry analyser (Abbott Diagnostics, Lake Forest [IL], US) was used to provide analysis for serum cholesterol, triglycerides, HDL, low-density lipoprotein (LDL), and fasting blood sugar (FBS). Fasting serum insulin (FINS) was analysed by chemiluminescence in a Centaur immunoassay (Bayer AG, Leverkusen, Germany).
Insulin Resistance
The basal-state method of Li et al9 was used for assessment of insulin resistance. The results were expressed as insulin sensitivity index (ISI). The ISI is the natural logarithm of the product of FINS and FBS (ISI = In [1/FBS × FINS]).
Genomic DNA Extraction
A 5-mL sample of peripheral venous blood was obtained from each patient, and sodium citrate was injected as an anticoagulant. Phenols were extracted by conventional methods and conventional phenol-chloroform methods were used to extract genomic DNA.
Determination of Paraoxonase-2-311 Polymorphism
Polymerase chain reaction (PCR)–restriction fragment length polymorphism analysis was used to determine gene polymorphisms. The primers used were: 5-GTG ACA TGC ATG TAC GGT GGT CT-3 and 5-ACA AGG CTC TGT GGT ATA AAG TGC C-3. The PCR volume was 25 μL comprising Taq DNA polymerase 0.2 μL (5 U/μL), 10x PCR buffer (Mg2+ buffers) 2.5 μL, deoxyribonucleotide triphosphate mix (each 2.5 mmol/L) 2 μL, template DNA (≤ 2.5 ng) 2 μL, each primer (10 pmol/μL) 0.5 μL, and double-distilled water up to 25 μL. The PCR reaction cycle was 94℃ for 5 minutes, followed by 94℃ for 30 seconds, 57℃ for 45 seconds, and 72℃ for 60 seconds (38 cycles). Then, the PCR product was held at 72℃ for 7 minutes, and cryopreserved at 4℃. The fragment size of the PCR product was 197 bp. The restriction enzyme double determinant enzyme immunoassay 5 U was added to the PCR product to create digestion reactions. The reactions were incubated in a water bath at 37°C for 4 to 16 hours. When the incubation was complete, 6x loading buffer 5 μL was added to each sample. Electrophoresis was done in 8% polyacrylamide gel at a constant 180 V for 3 hours, followed by silver nitrate staining and classification. The homozygous S/S yields were 78 bp and 70 bp, the homozygous C/C yields were 148 bp, and the heterozygous C/S yields were 148 bp, 78 bp, and 70 bp.10
Clinical Neuropsychological Assessment
The neuropsychological assessment was done to evaluate the clinical features among the 3 groups. The assessment included the Mini-Mental State Examination for screening cognitive function, Activities of Daily Living scale (ADL) to assess the extent to which the neurobehavioral impairment interfered with ADL tasks, Hachinski Ischemic Scale to ascertain the differences between vascular dementia and senile dementia, and Hamilton Depression Scale to assess depressive symptoms. The ratings were established by 2 psychiatry residents who had been trained before the study and had test results of more than 95% consistency.
Statistical Analysis
The Statistical Package for the Social Sciences Windows version 16.0 (SPSS Inc., Chicago, [IL], US) was used for statistical analysis. The measurement results of various data were expressed in mean ± standard deviation (SD). Analysis of variance was used to compare the measurement data between the groups. The count data and ranking data were compared using Chi-square test. Chi-square test was also used for allele and genotype frequencies. The results were considered statistically significant if the p value was less than 0.05.
Results
Demographics and Clinical Parameters
The demographics and clinical parameters of the 3 groups are shown in Table 1. The mean age of the presenile dementia patients was significantly lower than that of the other 2 groups (p < 0.01). There were no significant differences between the 3 groups for the remaining data (p > 0.05).
Since there were only 7 participants in the presenile dementia group, the number of patients was too small for meaningful analysis. Therefore, comparisons of some of the data were only made between the senile dementia and mixed dementia groups.
Neuropsychological Assessment
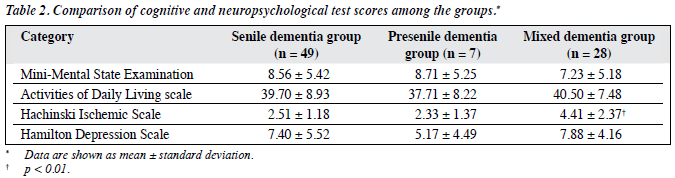
The Hachinski Ischemic Scale scores of the mixed dementia group were significantly higher than those of the presenile dementia and senile dementia groups (p < 0.01; Table 2).
Blood Biochemistry and Insulin Sensitivity Index
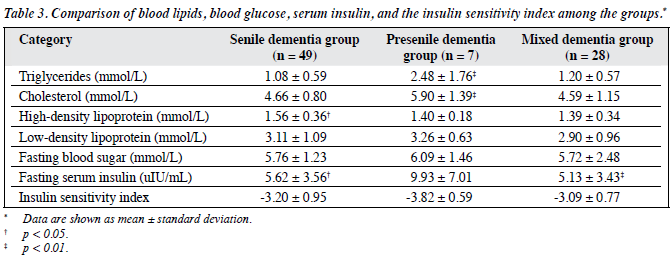
The triglyceride and cholesterol levels in the presenile dementia group were significantly higher than those of the senile dementia and mixed dementia groups (p < 0.01). The HDL level in the senile dementia group was significantly higher than that of the mixed dementia group (p < 0.05). The serum insulin level in the presenile dementia group was significantly higher than those of the senile dementia (p < 0.05) and mixed dementia groups (p < 0.01). There were no significant differences between the groups for the remaining results (Table 3).
Paraoxonase-2-311 Polymorphism and Allele Frequency
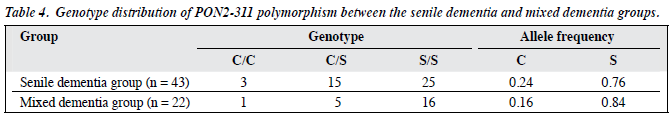
The differences in genotype distribution of PON2-311 polymorphism and allele frequency between the senile dementia group and mixed dementia group were not significant (p > 0.05; Table 4).
Discussion
The commonly used diagnostic criteria for AD include the ICD-10, DSM-IV, NINCDS-ADRDA, and CCMD-3. The CCMD-3 is most widely used for psychiatric diagnosis in China. Alzheimer’s disease is divided into 3 types of presenile, senile, and mixed dementia. The classification is based on age at onset, clinical features, and presence or absence of symptoms that also meet the criteria for a diagnosis of vascular dementia. The chronological age of 65 years at onset is the division between presenile and senile dementia. For mixed dementia, the symptoms are consistent with the diagnostic criteria for AD, but with atypical clinical manifestations, for example, disease onset after the age of 65 years with clinical manifestations of presenile dementia or the symptoms consistent with a diagnosis of cerebrovascular disease. This makes it difficult to establish a differential diagnosis. In this study, there were no significant differences in disease duration between the 3 groups. However, the mean (± SD) age of the presenile dementia group was 62.57 ± 2.88 years, which was significantly lower than those of the other 2 groups, and met the diagnostic criteria. Also worth noting is that this result suggests a relatively early onset of presenile dementia for these patients. The Hachinski Ischemic Scale score of the mixed dementia group was significantly higher than those of the presenile and senile dementia groups (p < 0.01), which is also consistent with the CCMD diagnostic criteria. The Hachinski Ischemic Scale is widely used to identify vascular dementia and AD; a higher score indicates a greater likelihood of developing vascular dementia. The results of this study suggest that the mixed dementia patients have characteristics of both AD and vascular dementia. The characteristics of vascular dementia include acute onset of disease, ladder-like pattern of disease progression, a history of stroke or atherosclerosis, focal neurological signs, confusion at night, loss of personal integrity, and emotional vulnerability.
Cardiovascular risk factors are closely related to AD, and hyperlipidaemia is considered an independent risk factor for AD.11 Epidemiological data showed that hyperlipidaemia can occur 10 to 15 years earlier than AD.12 A systematic review and meta-analysis suggests that the relationship between total cholesterol level and old-age dementia is dependent on age, such that people with high total cholesterol levels in middle age have a higher risk of developing AD in old age, whereas the total cholesterol level in old age has no relationship or a negative correlation with dementia in old age.13,14 Other studies support this view.15,16 The level of intracellular cholesterol is related to amyloid-β precursor protein (APP) metabolism. In normal circumstances, the processing of APP in the body is primarily carried out by α- and γ-secretase to produce a soluble APPα. However, in AD patients, processing of APP is carried out primarily by β- and γ-secretase, which produces excessive Aβ and ultimately leads to neuritic plaque formation. When the cholesterol level is elevated, the activity of α-secretase is inhibited, thereby promoting processing of APP mediated by β-secretase. The results of this study show that the levels of triglyceride and cholesterol in the presenile dementia group were significantly higher than that of the senile and mixed dementia groups (p < 0.01), while the HDL level in the senile dementia group was significantly higher than that of the mixed dementia group (p < 0.05). However, the results of current research into blood cholesterol levels in AD are not consistent.17,18 The reason for the inconsistency may be that the types of AD in the study participants varied between studies, which suggests that, when selecting patients for AD research, it is important to ensure a uniform subtype diagnosis. It is known that HDL plays a role in protection against cerebrovascular diseases. The results of this study suggest that the cardioprotective effects of HDL in the senile dementia group are greater than those of the mixed dementia group.
Insulin resistance refers to reduced glucose-lowering effects of insulin. When the body tries to maintain normal glucose homeostasis, the cells produce a compensatory increase in insulin secretion that may eventually lead to hyperinsulinaemia. Studies have shown that, by accelerating the formation of neurofibrillary tangles, hyperglycaemia and hyperinsulinaemia caused by insulin resistance may affect Aβ aggregation, abnormal phosphorylation of tau protein, and induction of inflammation — all mechanisms that promote the onset of AD. Insulin and insulin-like growth factors play an important role in neuronal survival related to learning and memory in humans, energy metabolism, and plasticity.19-21 Du et al22 conducted a study using in- vitro cultured nerve cells and dementia models of rats. These authors believe that Aβ may cause neuronal damage and induce learning and memory dysfunction. These mechanisms of neural impairment are mediated by defects in the insulin signal transduction pathway and hippocampal neuronal dysfunction. One study has suggested that AD might be a specific form of diabetes — namely type 3 diabetes — that is associated with insulin deficiency and insulin resistance confined to the central nervous system rather than peripheral insulin resistance.23 The results of this study show that the serum insulin level in the presenile dementia group was significantly higher than that of the senile dementia (p < 0.05) and mixed dementia (p < 0.01) groups. However, the insulin sensitivity indices among the 3 groups were not significantly different, suggesting no significant differences between the 3 groups in peripheral insulin resistance.
Shi et al4 researched the PON2 gene in 165 sporadic AD patients and 174 healthy controls. These authors inferred that PON2-311C in Han Chinese AD patients was independent of the risk factor APOE ε4. However, Wu and Qian24 did not find a correlation between PON2- 311C/S polymorphism and AD. A study by Zhang et al25 of 110 cerebral infarction patients and 100 healthy controls indicates that the G allele of PON2-311C/S might be a risk factor for cerebral infarction in the Han Chinese population in the Shanxi region of China. A study of 22 AD patients and 31 age-matched healthy controls by Wu et al8 concluded that in Hainan, China, no significant correlation was found between PON2-311C/S polymorphism and AD; there was also no significant correlation between the patients and the blood lipids or glycaemic index. Additionally, there were no statistical differences in PON2-311 allele frequencies or genotype distribution between patients with one of 2 subtypes of AD. These results suggest that there is no significant difference in genetic polymorphisms between senile dementia and mixed dementia patients with AD. The changes in lipoprotein metabolism and the anomalies caused by oxidative stress were considered to be major causes of AD. As an antioxidant enzyme, PON may play a key role in protecting the body from toxic elements.26 The PON2 can reduce the level of intracellular oxidative stress to prevent cell-mediated LDL oxidation. The PON2 may also affect the process of apoptosis and atherosclerosis by combining with PON1 and PON3 for a joint effect in reducing the detrimental actions of vascular oxygen-free radicals. Also worth noting is that oxidative stress as a cause of complex diseases such as AD has been considered an important hypothesis.27 Bourquard et al28 noted that PON2 plays an important role in hepatic insulin signalling in animal liver and affects the influence of macrophage- mediated inflammatory response on hepatic insulin sensitivity. However, the effects of PON2 on the insulin signalling system in the brain are still unclear.
Since AD is a complex disease, there are limitations to this study. Many factors are thought to play a role in AD, including diet, physical activity, and co-morbid physical illness. These confounding factors may also affect blood lipids and insulin metabolism. Moreover, the CCMD-3 diagnostic criteria has limitations in that it may include some non-AD dementias such as primary progressive aphasia caused by frontotemporal lobar degeneration or vascular brain injury caused by non-Aβ pathological changes in the arteries. In 2011, the National Institute on Aging-Alzheimer’s Association recommended a new set of diagnostic criteria for AD.29 The guideline emphasises the importance of biomarkers for early detection of AD, and clearly states that AD is a continuous pathophysiological process, which includes the early phases of pre-mild cognitive impairment and mild cognitive impairment, and the later stage of dementia. The combination of 2 diagnostic criteria will allow for better understanding of the disease and improve diagnostic accuracy.
In summary, this research demonstrated no significant difference in PON2-311C/S polymorphism between senile dementia and mixed dementia AD patients diagnosed according to the CCMD-3 criteria. The study demonstrated that there were some differences in lipid composition and insulin metabolism among the 3 subtypes of AD. The results suggest that attention should be paid to monitoring of, and interventions for, blood glucose and insulin metabolism in AD patients with presenile dementia to help prevent and treat the disease.
Acknowledgement
The foundation project was funded by the Guangdong Provincial Natural Science Foundation (No. 8151037001000006), a pharmaceutical and health science technology project of the Guangzhou Municipal Government (2007-YB-104).
References
- Chinese Medical Association. Chinese classification of mental disorders. 3rd ed (CCMD-3) [in Chinese]. Jinan: Shandong Science and Technology Press; 2001: 32-4.
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 1997;278:1349-56.
- Pasdar A, Ross-Adams H, Cumming A, Cheung J, Whalley L, St Clair D, et al. Paraoxonase gene polymorphism and haplotype analysis in a stroke population. BMC Med Genet 2006;7:28.
- Shi J, Zhang S, Tang M, Liu X, Li T, Han H, et al. Possible association between Cys311Ser polymorphism of paraoxonase 2 gene and late- onset Alzheimer’s disease in Chinese. Brain Res Mol Brain Res 2004;120:201-4.
- Klimkowicz-Mrowiec A, Marona M, Wolkow P, Witkowski A, Maruszak A, Styczynska M, et al. Paraoxonase gene polymorphism and the risk for Alzheimer’s disease in the polish population. Dement Geriatr Cogn Disord 2011;31:417-23.
- Janka Z, Juhász A, Rimanóczy AA, Boda K, Márki-Zay J, Kálmán Codon 311 (Cys->Ser) polymorphism of paraoxonase-2 gene is associated with apolipoprotein E4 allele in both Alzheimer’s and vascular dementias. Mol Psychiatry 2002;7:110-2.
- Xu HW, Zhao Z, Yuan N, Xiao B, Yang XS, Tang BS. Relationship between single nucleotide polymorphisms of paraoxonase 2 and stroke [in Chinese]. Chin J Med Genet 2007;24:328-30.
- Wu L, Li YL, Wu Q, Chen SJ, Mai Z. The association of the paraoxonase-2 311Cys/Ser polymorphism and blood lipids and blood glucose index with Alzheimer’s disease in Hainan [in Chinese]. J Mol Diagn Ther 2012;4:253-6.
- Li GW, Pan XR, Lillioja S, Bennett PH. A new insulin-sensitivity index for the population-based study [in Chinese]. Zhonghua Nei Ke Za Zhi 1993;32:656-60.
- Liang HY, Wu BY, Chen DF, Yang F, Hu HY, Chen L, et al. Association of CYP2E1 and PON2311 polymorphisms in neonates with preterm [in Chinese]. Yi Chuan Xue Bao 2002;29:847-53.
- Chou CX. Cardiovascular risk factors in Alzheimer’s disease patients: pathological mechanisms and prevention interventions in a general population [in Chinese]. J Jining Med Univ 2012;35:78-83.
- Kivipelto M, Helkala EL, Laakso MP, Hänninen T, Hallikainen M, Alhainen K, et al. Midlife vascular risk factors and Alzheimer’s disease in late life: longitudinal population based study. BMJ 2001;322:1447- 51.
- Solomon A, Kivipelto M. Cholesterol-modifying strategies for Alzheimer’s disease. Expert Rev Neurother 2009;9:695-709.
- Anstey KJ, Lipnicki DM, Low LF. Cholesterol as a risk factor for dementia and cognitive decline: a systematic review of prospective studies with meta-analysis. Am J Geriatr Psychiatry 2008;16:343-54.
- Rao DP, Tang MN, Ma C, Guo YB, Han HY, Huang XM, et al. Analysis of Alzheimer’s disease, vascular dementia, and lipid concentrations [in Chinese]. J Clin Psych 2008;18:220-2.
- Mu N, Xu SC, Chang Q, Chen JP, Liu XJ, Ma C. A clinical study of insulin resistance serum lipid and cortisol in patients with sporadic Alzheimer’s disease [in Chinese]. Guangzhou Med J 2010;41:3-5.
- Solomon A, Kivipelto M, Wolozin B, Zhou J, Whitmer RA. Midlife serum cholesterol and increased risk of Alzheimer’s and vascular dementia three decades later. Dement Geriatr Cogn Disord 2009;28:75- 80.
- Borroni B, Colciaghi F, Lenzi GL, Caimi L, Cattabeni F, Di Luca M, et High cholesterol affects platelet APP processing in controls and in AD patients. Neurobiol Aging 2003;24:631-6.
- Matsuzaki T, Sasaki K, Tanizaki Y, Hata J, Fujimi K, Matsui Y, et Insulin resistance is associated with the pathology of Alzheimer disease: the Hisayama Study. Neurology 2010;75:764-70.
- Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol 2006;5:64-74.
- Schrijvers EM, Witteman JC, Sijbrands EJ, Hofman A, Koudstaal PJ, Breteler MM. Insulin metabolism and the risk of Alzheimer disease: the Rotterdam Study. Neurology 2010;75:1982-7.
- Du YF, Yan P, Han XJ. Study on the impairment of insulin signal transduction pathway in Alzheimer’s disease [in Chinese]. J Jining Med Univ 2012;2:84-92.
- de la Monte SM. Insulin resistance and Alzheimer’s disease. BMB Rep 2009;42:475-81.
- Wu Q, Qian SY. The association of paraoxonase 2 polymorphisms with Alzheimer’s disease [in Chinese]. Chin J Gerontol 2007;27:1038-41.
- Zhang ST, Li DF, Zhang HP, Pei YH, Lian X, Liu CY. Correlations: paraoxonase 2 (PON2) polymorphisms and cerebral infarction [in Chinese]. Chin J Integr Med Cardio-Cerebrovasc Dis 2012;6:686-8.
- Erlich PM, Lunetta KL, Cupples LA, Huyck M, Green RC, Baldwin CT, et al. Polymorphisms in the PON gene cluster are associated with Alzheimer disease. Hum Mol Genet 2006;15:77-85.
- Sheng SL. Alzheimer’s disease and related disorders [in Chinese]. Beijing: Scientific and Technology Literature Publishing House; 2006: 556-66.
- Bourquard N, Ng CJ, Reddy ST. Impaired hepatic insulin signalling in PON2-deficient mice: a novel role for the PON2/apoE axis on the macrophage inflammatory response. Biochem J 2011;436:91-100.
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011;7:280-92.