East Asian Arch Psychiatry 2015;25:3-15
ORIGINAL ARTICLE
强迫症患者其抑郁症共病对神经认知功能的影响:印度研究
Dr Dharmendra Singh, MD, Department of Psychiatry, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
Prof. Surendra K. Mattoo, MD, Department of Psychiatry, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
Dr Sandeep Grover, MD, Department of Psychiatry, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
Prof. Adarsh Kohli, MA, PhD, Department of Psychiatry, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
Address for correspondence: Dr Sandeep Grover, Department of Psychiatry, Postgraduate Institute of Medical Education and Research, Chandigarh 160012, India.
Tel: (91-172) 2756807; Fax: (91-172) 2744401 / 2745078; Email: drsandeepg2002@yahoo.com
Submitted: 22 April 2014; Accepted: 4 July 2014
Abstract
Objective: To study neuropsychological function in subjects with obsessive-compulsive disorder (OCD) with and without co-morbid depression in comparison with healthy controls (HC).
Methods: The 3 sample groups included subjects with OCD without depression (OCD group, n = 30); subjects with OCD and depression (OCDD group, n = 20); and HC (n = 25). All 3 groups were matched for age, gender, and years of education, and they were assessed on the following: Trail Making Tests A and B, Verbal Fluency Test, PGI Memory Scale, Stroop test, Tower of London Test, Raven’s Standard Progressive Matrices, Wisconsin Card Sorting Test, and the Object Alternation Test.
Results: Both OCD and OCDD groups performed more poorly than HC, whereas the OCDD group performed worse than OCD group. Besides, OCD and OCDD groups had significantly poorer performance on tests of attention, memory, executive functions, verbal fluency, and intelligence. The OCDD group performed worse than the OCD group notably on Verbal Fluency Test, PGI memory test, and Object Alternation Test.
Conclusion: On tests of neurocognitive functioning, the performance of the OCDD group was poorer than the OCD group, and both performed poorer than HC, suggesting that OCD is associated with neurocognitive dysfunction and that this is exacerbated in the presence of depression.
Key words: Cognition disorders; Depression; Obsessive-compulsive disorder
摘要目的:有或没有抑郁症共病的强迫症患者,其神经心理功能与对照组作较。
方法:研究对象共3组,包括没有抑郁症共病的强迫症患者(OCD组,30人)、有抑郁症共病的强迫症患者(OCDD组,20人),以及健康对照组(25人)。三组均根据年龄、性别和教育程度进行配对并接受下列评估,包括路径描绘测验、词语流畅性测验、PGI记忆量表、Stroop色字测验、伦敦塔测验、瑞文推理测验、威斯康星卡片分类测验以及物件交替测验。
结果:两组患者的表现皆比对照组差,而OCDD组的表现也比OCD组差。两组患者的注意力、记忆力、执行功能、言语流畅性和智力的表现都显着较差,而OCDD组在词语流畅性测验、PGI记忆量表和物件交替测验的表现明显较OCD组差。
结论:OCDD组的神经认知功能测试比OCD组差,而两组患者的表现又比健康对照组差。这表明强迫症与神经认知功能障碍相关。抑郁共病也可使情况恶化。
关键词:认知障碍、抑郁症、强迫症
Introduction
Assessment of cognitive functions in obsessive-compulsive disorder (OCD) is increasingly recognised as a valid approach to understanding the underlying neuroanatomical substrates. Cognitive functions studied have included attention, executive functions, visuospatial functions, verbal and non-verbal memory, as well as intelligence.1 However, there has been no consensus about the dysfunctional components associated with this disorder, as compared with healthy control subjects. Some studies reported no difference in reaction time, alertness tasks and speed of information processing,1-4 while others reported impaired speed of information processing.5-7 Studies assessing both attention span and sustained attention by using the Revised Wechsler Adult Intelligence Scale (WAIS-R) Digit Span Forward subtest reported no impairment in subjects with OCD.2,4,8 While some studies showed impairment in focused attention and distractibility9,10 in comparison to patients with panic disorder and healthy control subjects, later research failed to replicate these findings.2,5,10,11
Assuming an underlying fronto-striatal loop dysfunction,12 patients with OCD have been examined for executive functions, such as set shifting ability, fluency, conceptual thinking, and planning abilities. Comparison studies with healthy control subjects using the Wisconsin Card Sorting Test (WCST) for testing attentional set shifting ability have reported either no differences,8,12,13 or impaired performance in those with OCD.14,15 Brain imaging studies have documented aberrant hyperactivity of pathways in the orbitofrontal cortex, caudate nucleus, and anterior cingulate cortex in OCD.16 As the WCST performance is being supposedly most affected by dorsolateral prefrontal cortex lesions, its validity of assessing cognitive impairments in OCD is questioned. Hence, it is suggested using tests which may more specifically identify malfunction of the orbitofrontal cortex, caudate nucleus, and anterior cingulate cortex. The Object Alternation Test (OAT) and the Delayed Alternation Test, considered to be more sensitive for orbitofrontal damage,17,18 have been reported to reveal marked deficits in OCD in many,19,20 but not all, studies.21 A review of various studies comparing healthy control subjects and individuals with OCD for fluency22 found those with OCD scored significantly lower in 7 studies, no difference in 9 studies, and those with OCD scored better in 1 study. In contrast, comparison studies with healthy control subjects using the Tower of London (TOL) test for conceptual thinking and planning ability showed deficits in those with OCD, specifically the inability to generate alternative strategies following an incorrect move,23 and significant differences in initial movement time.24 However, these findings have not been replicated.5,19,25 Instead, studies evaluating visuospatial ability suggest dysfunction in one or the other subtest.4,13,26
While studies comparing non-verbal memory in patients with OCD and healthy control subjects have shown impaired immediate recall in those with OCD,27,28 a comparison study between drug-naïve OCD subjects and healthy control subjects showed no difference in non-verbal memory skills.29 Although older studies have not reported deficits in verbal memory in patients with OCD, some recent studies have reported impaired verbal memory as assessed by tests requiring presentation of stimuli in a semantically clustered fashion, such as the California Verbal Learning Test13,15 or other verbal memory tasks.21 Other studies have shown general intelligence as unaffected in OCD or that differences between patients with OCD and healthy control subjects are small and non-significant.5
Most of the cited research is limited by variability in methodology, sample size, demographic variables and neurocognitive tests, as well as non-evaluation of the effect of co-morbidity and the severity of illness on cognitive functioning.22 Thus, there is no consensus, and a large scope for studies with comprehensive neuropsychological batteries for greater specificity. The present research aimed to study neuropsychological functions in subjects with OCD, with and without co-morbid depression, in comparison with healthy control subjects. It also aimed to examine the relationship between neurocognitive functions and specified socio-demographic and clinical variables.
Methods
The study was conducted at the Department of Psychiatry, Postgraduate Institute of Medical Education and Research, Chandigarh, India. As an MD thesis of the first author it had the Institute Ethics Committee approval. Subject recruitment was based on written informed consent. With a cross-sectional design, assessments were completed in 1 to 2 sessions within 48 hours. The sample comprised 2 patient groups, one with OCD without depression (OCD group, n = 30) and the other with OCD plus depression (OCDD group, n = 20) according to the diagnostic criteria of the Mini- International Neuropsychiatric Interview (MINI),30 as well as healthy controls (HC, n = 25). The HC were recruited from non–blood-related attendants accompanying the OCD patients. The 3 groups were matched for age, gender, and years of education.
For the OCD groups, the inclusion criteria were: diagnosis by the DSM-IV,31 diagnosis of OCD as per the MINI,30 aged 18 to 60 years, had received primary education, and not on benzodiazepines 2 weeks prior to assessment. Exclusion criteria were OCD with either co-morbid psychiatric disorders (except depression), chronic physical illness, alcohol / substance dependence (except for nicotine dependence) or organic brain syndromes, and those who received electroconvulsive therapy in the past 6 months.
For the HC, the inclusion criteria were: subjects aged 18 to 60 years, had received primary education, no personal history of psychiatric illness (except for nicotine dependence), and no family history of psychiatric illness in first-degree relatives. Exclusion criteria were chronic physical illness, alcohol or substance dependence (except for nicotine dependence), organic brain syndromes, and psychiatric illness in first-degree relatives.
Assessment Tools
The MINI30 was used to diagnose OCD and depressive disorder and to rule out other co-morbid disorders. The Hamilton Depression Rating Scale32 (HDRS) was used to rate severity of depression. The Yale-Brown Obsessive Compulsive Scale33 (YBOCS) was used to rate the severity of OCD.
Neurocognitive Test Batteries
The WCST34 measured the ability to form abstract concepts, shift and maintain set, utilised feedback and the inhibitory control of interference, all thought to be primarily mediated by the dorsolateral prefrontal cortex.
Regarding Trail Making Tests A and B (TMT-A, TMT-B),35 TMT-A involved drawing lines on a sheet of paper to connect consecutively numbered circles from 1 to 25, while TMT-B involved drawing lines to connect 25 consecutively numbered and lettered circles by alternating between the 2 types of sequences. Trail Making Tests assessed speed of attention, sequencing, mental flexibility, visual search, and motor function. Time taken on TMT-A and TMT-B, respectively, reflected visual searching ability versus processing speed and mental tracking, and TMT-B error scores reflected working memory and executive functioning.35
Developed at our Centre from the Wechsler Memory Scale, the PGI Memory Scale (PGIMS, Hindi version)36 consisted of 9 verbal and 1 non-verbal subtests of memory. It measured remote memory, recent memory, mental balance, attention and concentration, delayed recall, immediate recall, retention for similar pairs, retention for dissimilar pairs, visual retention, and recognition. As a standardised test, it took 15 to 20 minutes to administer, provided age- and education-based norms for the Indian population, had a test-retest reliability of 0.69 to 0.85, and correlated highly with scores on the Boston Memory Scale and the Wechsler Memory Scale (0.71 and 0.85, respectively).36
The Raven’s Standard Progressive Matrices (RSPM)37 measured Spearman’s general intelligence factor in those aged 6 to 80 years, using 5 black and white sets of 12 problems each, and taking 20 to 45 minutes to complete. The total number of problems solved correctly was the total score. The WAIS-equivalent intelligence quotient (IQ) was determined from the tabulated raw scores, using a prescribed table.
The Stroop test38 was used as a measure of response inhibition. It contained capital letters printed on a paper in different colours. The colour print did not correspond with the colour designated by the words. The words were printed in 16 rows and 11 columns and the test could be completed in around 20 minutes. The stimulus sheet was placed in front of the subject. In the first trial, they were asked to read the word and in the second trial, name the colour in which the word was printed. They should read it column-wise. Timings were recorded for both trials. The reading time was subtracted from the naming time to get the Stroop test effect score. Uncorrected errors were noted separately for both phases.
The Verbal Fluency Test (VFT)39 required the participants to respond, usually in 1 minute, to usual stimulus letters ‘K’, ‘P’, and ‘M’ (which were used as substitutes for ‘F’, ‘A’, and ‘S’ in Hindi, respectively) with as many words as possible from a category (avoiding proper nouns and antonyms). As a test of mental flexibility, it was thought to involve the prefrontal cortex.
The TOL test40 evaluated the ability to plan and anticipate the results of one’s actions to achieve a predetermined goal. It consisted of 2 identical wooden boards, each fitted with 3 round pegs of different sizes and 3 wooden balls in red, green, and blue; going by respective height, the 3 pegs could hold 3, 2, and 1 balls each. The subject needed to solve certain problems for which the time taken from start to finish was noted. The test score per problem was the number of moves (the subject lifting the ball). The usual completion time was about 30 minutes.
The OAT41 evaluated the ability of an individual to shift sets and working memory for objects. The apparatus used was a 55 cm wide and 65 cm high wooden frame, a black curtain anchored to it, and a board containing 2 reinforcement wells covered by a different three- dimensional stimulus object. The objects differed in shape and colour, and one of them had a coin underneath. In multiple trials, the subject had to choose the object with the coin underneath. To do that successfully, he / she had to learn that the location of the object with the coin underneath was being alternated after each correct response.
The Edinburgh Handedness Inventory42 was used to assess the hand preference / handedness. It covered 10 different activities, including writing, drawing, throwing, scissor use, toothbrush use, knife cutting, spoon use, broom use, striking a match, and opening a box. The Handedness Index was calculated as: total R – total L × 100 / total R + total L (‘total R’ referred to activities done with right hand while ‘total L’ referred to activities done with left hand).
Each participant completed the tests in the same order, including the TMT-A, TMT-B, VFT, PGIMS, Stroop test, TOL test, RSPM, WCST, OAT, and Edinburgh Handedness Inventory. Assessment was completed over 90 to 120 minutes in 1 to 2 sessions within 48 hours. All medications were withheld for 12 hours prior to the testing to prevent any interference in performance.
Statistical analysis was completed using the SPSS version 14.0. Descriptive analysis used the mean and standard deviation with the range for continuous variables, and the frequency and percentages for discontinuous variables. Comparisons were done using the t test, Mann- Whitney test, analysis of variance (ANOVA), and Chi- square test. Correlations were studied using Pearson’s product moment correlation and Spearman’s rank correlation analyses. Univariate and multivariate analyses of covariance (ANCOVA, MANCOVA) were applied to control for the effect of current IQ, residual depression, severity of OCD, and duration of illness on the neuropsychological parameters.
Results
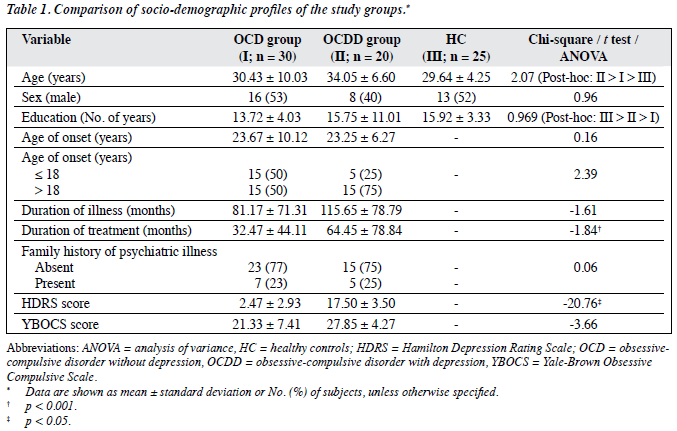
As the study design required, all 3 groups were matched for gender, years of education, and age (mean age being around 30 years). The OCD group and the HC had a near-equal gender distribution, while 60% of the OCDD group were females; the difference was insignificant. The mean years of education in the 3 groups ranged from 13.72 to 15.92 years (Table 1).
The 2 patient groups did not differ significantly for mean age of onset, number of subjects with age at onset of illness ≤ 18 years, duration of illness, family history of psychiatric illness, and mean YBOCS score. The OCDD group was on treatment for a significantly longer duration than the OCD group; as expected, the OCDD group also had a significantly higher HDRS score (Table 1).
Neurocognitive Profiles
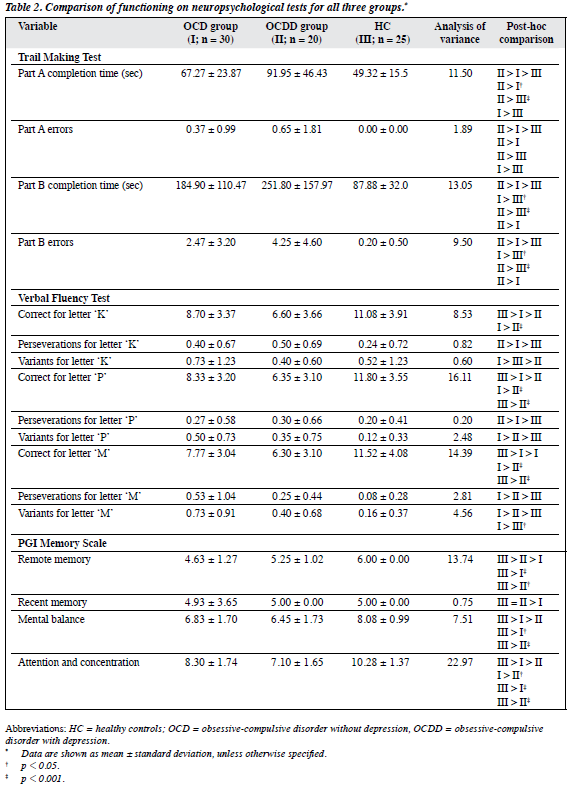
On neurocognitive tests both patient groups performed worse than the HC, while the OCDD group performed worse than the OCD group.
Compared with the OCD group, the OCDD group took significantly more time to complete TMT-A and gave significantly less numbers of correct ‘K’, ‘P’ and ‘M’ responses on the VFT. The OCDD group also had significantly poorer attention and concentration, immediate recall, retention of dissimilar pairs for new learning and overall memory on the PGIMS, the number of problems solved in minimum moves in trial 3 on the TOL test, and committed more total number of errors and more errors for subjects on the left side on OAT. The 2 patient groups performed similarly on the WCST, handedness, and intelligence. In all, more significant differences were noted in tests for comparison of OCDD versus HC than OCD versus HC (Table 2).
Covariate Analysis
Comparison of Neuropsychological Performance
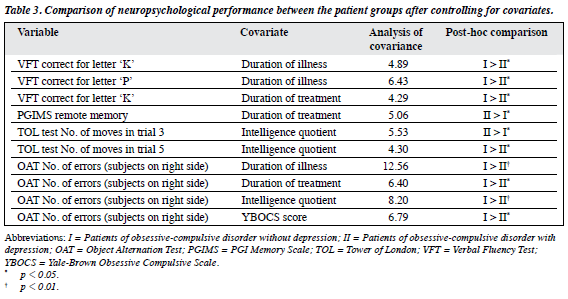
Based on the significant differences between OCD and OCDD groups, duration of illness, duration of treatment, IQ, and YBOCS score were used as covariates to compare neuropsychological functioning. Additionally, based on the literature evidence for some of neuropsychological tests for which there was no significant difference, ANOVA and ANCOVA were used for these variables. With duration of illness, duration of treatment, IQ, and YBOCS score as covariates, the difference between the patient groups on VFT and OAT was significant. With duration of treatment as a covariate, the difference on VFT correct for letter ‘K’, OAT number of errors in subjects on right side, and PGIMS remote memory between the patient groups was significant. With IQ as a covariate, some subtests of the TOL test were significant between the patient groups. With YBOCS score as a covariate, the difference between the patient groups was significant on the OAT number of errors in subjects on right side (Table 3).
However, when all these 4 variables (duration of illness, duration of treatment, IQ, and YBOCS score) and total HDRS score were used as multiple covariates, the patient groups showed significant difference only on PGIMS remote memory (MANCOVA F = –4.28, p < 0.05).
Further covariate analysis was done for the influence of depression (HDRS score) on the neurocognitive functioning of both patient groups. For all the neurocognitive tests for which there was a significant difference between the patient groups, ANCOVA was done with HDRS as a covariate. After controlling for HDRS score, the significant differences between the patient groups remained on OAT total number of errors (ANCOVA F = –13.20, p < 0.01) and OAT number of errors for subjects on left side (ANCOVA F = –18.20, p < 0.001). For further analysis, 4 variables (duration of illness, duration of treatment, IQ, YBOCS score) were used as covariates and the significant difference between the patient groups on OAT total number of errors (MANCOVA F = –13.18, p < 0.01) and OAT number of errors for subjects on left side (MANCOVA F = –20.26, p < 0.001) were still evident. However, when all covariates (duration of illness, duration of treatment, IQ, YBOCS and HDRS scores) were analysed together, there was no significant difference between the patient groups on these subtests.
Analysis of covariance was also done for the influence of HDRS on the neuropsychological functions which did not differ between the patient groups on ANOVA. Using HDRS as a covariate, the difference between the patient groups was significant for the TMT-B completion time (ANCOVA F = –5.10, p < 0.05), TOL test number of moves in trial 4 (ANCOVA F = –5.65, p < 0.05), and TOL test number of problems solved in minimum moves in trial 5 (ANCOVA F = –4.33, p < 0.05). However, when MANCOVA was done using all the 5 covariates (duration of illness, duration of treatment, IQ, YBOCS and HDRS scores), no significant difference was found between the patient groups on these subtests, except for TMT-B completion time (MANCOVA F = 6.10, p < 0.05).
Relationship of Neuropsychological Functioning with Different Variables
Socio-demographic Variables
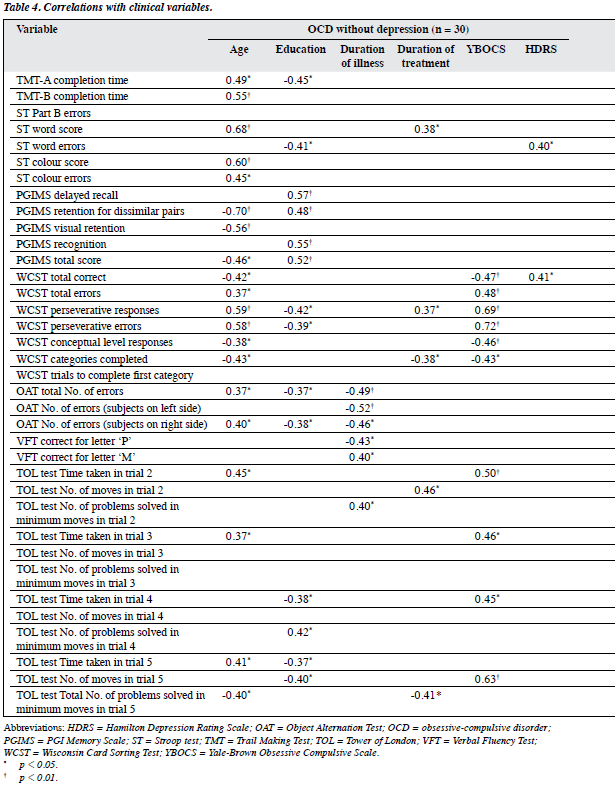
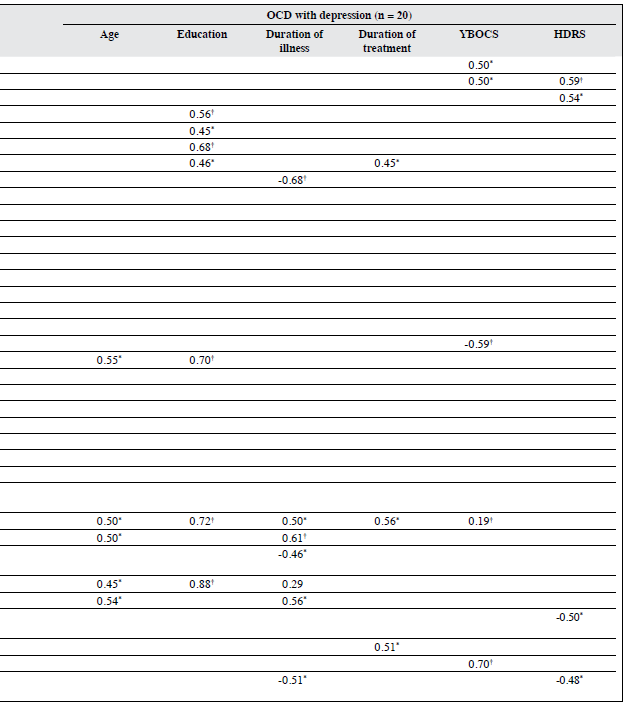
Significant correlations emerged between age and years of education and various neurocognitive functions in both patient groups (Table 4).
Clinical Variables
Regarding OCD group, longer duration of illness was associated with better functioning on the OAT and VFT (for letter “P” responses), but poorer performance in terms of VFT for letter “M” responses and fewer problems solved in minimum moves in trial 2 on the TOL test. Longer duration of treatment was associated with significantly lower word scores on the Stroop test, more perseverative responses and fewer categories completed on the WCST. Greater severity of psychopathology (on YBOCS) was associated with poorer performance on the WCST, as well as more time taken in the completion of trials 2 to 4 and more moves needed in trial 5 on TOL test. Besides, residual depressive symptoms (on HDRS) correlated with word errors on the Stroop test and better performance on the WCST total number of correct responses (Table 4).
For OCDD group, longer duration of illness had a positive correlation with better performance on the PGIMS delayed recall and poorer performance trial 3 on the TOL test. Longer duration of treatment was associated with significantly more colour errors on the Stroop test, and longer time taken in completion of trials 3 and 5 of the TOL test. Greater severity of psychopathology (on YBOCS) was associated with poorer performance on the TMT and better functioning on some of the subtests of the WCST (number of categories completed), and poorer performance on the TOL test, in the form of more time taken in the completion of trial 3 and more moves on trial 5. More severe depressive symptoms were associated with poorer performance on the TMT-B and higher number of Part B errors on the Stroop test (Table 4).
Discussion
Neurocognition is being increasingly recognised as a valid tool to unravel the neuroanatomical substrates underlying OCD. As an intermediate path between clinical assessment and functional imaging studies, neurocognitive research in OCD has focused on attention, executive functions, visuospatial functions, and verbal and non-verbal memory,2-4 to provide clinical researchers with a better understanding of OCD. However, the final word on the pattern of neurocognitive deficits in OCD is still awaited, mainly due to the methodological limitations of existing research. Our research has advanced the research approach by comparing neurocognitive functions in patients with OCD, with and without depression, and HC matched on age, gender, years of education, and handedness.
So far only a few studies have compared OCD with and without depression,6 and these did not match the 2 groups for variables such as age of onset and duration of illness, which can influence cognitive functioning. In contrast, we matched our OCD groups for age of onset, duration of illness, family history of psychiatric illness, and severity of obsessive-compulsive symptoms on the YBOCS. Thus, the neurocognitive differences in our patient groups reflect the true influence of depression on the neurocognitive profile of OCD.
Although our OCD groups were not drug-naïve, the effect of medication was minimised by carrying out the assessment at least 12 hours after medication intake.
Neurocognitive Profile
In our study, attention was measured by the PGIMS, TMT, and Stroop test. Both patient groups displayed poorer attention (except on the TMT-A) than HC, and the difference was significant for the HC and OCDD group on all these tests. Besides, OCD group differed significantly from the HC on Part B completion time on TMT, some PGIMS subscales, and Stroop test. Previous research both support43 and contradict9,10 our finding. Studies using the WAIS-R and Digit Span Forward have also reported attention span and sustained attention as unaffected in OCD,1-3,5,44 but our findings on the PGIMS contradict these. Some studies using the Stroop test have reported impaired selective attention8 as was seen in the present study.
Although some studies in OCD report attention as unaffected, some recent studies10,12 and our study show attention as impaired. The discrepancy may be explained by many of the previous studies not controlling for some socio- demographic and clinical variables, which could influence performance on attention tasks. Also, very few studies have compared attention in OCD with and without depression. Our finding that when compared with the OCD group, the OCDD group showed significantly more impairment in attention as measured by the TMT, PGIMS, and Stroop test is supported by another study.7
Our finding of significantly impaired WCST which measured performance on set shifting in both patient groups supports5,11,45 and contradicts2,46,47 the existing literature. The inconsistency has prompted the suggestion that the OAT is a more appropriate test for set shifting ability.27 In our study, on the OAT both patient groups performed more poorly than the HC, and the OCDD group performed more poorly than the OCD group. Our finding of impairment in the patient groups versus HC is consistent with most of the existing literature.1,46,48-50 The OAT is considered very sensitive to orbitofrontal damage.48 The OAT deficits observed in this study support the neuroimaging and neuropsychological findings51,52 suggesting involvement of the orbitofrontal cortex in the pathogenesis of OCD.
Both patient groups performed poorly on the VFT, and significant differences were noted between OCDD group and HC in many subtests. This finding is consistent with some,22,45,53,54 but not other8 studies. The inconsistent findings may be due to the need to control for depression as a covariate.
We measured conceptual thinking and planning ability using the TOL test, the performance on which correlated with activation of the dorsolateral prefrontal cortex and additionally the premotor and parietal cortex.55 Our OCDD group performed significantly worst and the HC performed the best on this test; the intergroup differences were significant only for a few subtests. This latter finding is supported by a previous study.6
Both patient groups performing poorly on non-verbal memory tests, supporting other studies reporting deficits in the delayed recognition test2,8,44,45,56 and visual recognition tasks,5,10 though others44,57,58 reported no impairment of non- verbal memory. They also had significant deficits in PGIMS which measured verbal memory, the finding of which is supported29,56,58 as well as contradicted2,7,10,44,45,59 in the literature.
The patient groups assessed as having lower IQ scores compared with HC is not supported by the literature.5 However, this needs to be re-examined in view of other potential confounders, such as duration of treatment and duration of illness.
The findings of this study suggest that patients with OCD have impairment in attention, executive functions, conceptual thinking, planning, verbal fluency, non-verbal memory tasks, which are further worsened by the presence of depression. Accordingly, assessment of depression should never be ignored when assessing the neurocognitive function of patients with OCD. Findings of the present study also suggest that the WCST is able to identify executive dysfunction in patients with OCD. However, findings also suggest that the OAT is more sensitive to executive dysfunction than the WCST in the presence of depression and therefore may be more appropriate for the assessment of patients with OCD.
Correlates of Neurocognitive Functions
Our findings showed that while performance on most of the neurocognitive test was influenced by age and level of education, this was more marked in patients with OCD; use of case-control study design may therefore be helpful in future research. Our findings that in OCD without depression, performance on the OAT was influenced by duration of illness, and performance on the WCST was correlated with YBOCS severity score, are supported by previous research.14,60
Study limitations
Findings of our study must be interpreted in the context of its limitations in the form of a small sample size and one time assessment. We also did not evaluate the neuroimaging correlates of identified neuropsychological deficits. In addition, the study did not investigate the relationship between neurocognitive deficits and symptomatology. Future studies should attempt to overcome the limitations of our study.
Conclusion
Our findings suggest that in terms of neurocognitive function on standard assessment tools, HC performed better than patients with OCD, with or without depression. Furthermore, our study suggests that depression impairs neurocognitive functioning in OCD. Hence, future studies in this area should control for depression. In terms of clinical practice, the presence of neurocognitive deficits in various domains in patients with OCD must be evaluated and managed appropriately in order to improve the outcome and quality of life of these patients.
Declaration
The authors declared no conflict of interests in this study.
References
- Aronowitz BR, Hollander E, DeCaria C, Cohen L, Saoud JB, Stein D, et al. Neuropsychology of obsessive compulsive disorder: preliminary findings. Neuropsychiatry Neuropsychol Behav Neurol 1994;7:81-6.
- Berthier ML, Kulisevsky J, Gironell A, Heras JA. Obsessive-compulsive disorder associated with brain lesions. Clinical phenomenology, cognitive function, and anatomic correlates. Neurology 1996;47:353- 61.
- Cohen LJ, Hollander E, DeCaria CM, Stein DJ, Simeon D, Liebowitz MR, et al. Specificity of neuropsychological impairment in obsessive- compulsive disorder: a comparison with social phobic and normal control subjects. J Neuropsychiatry Clin Neurosci 1996;8:82-5.
- Jurado MA, Junqué C, Vallejo J, Salgado P. Impairment of incidental memory for frequency in patients with obsessive-compulsive disorder. Psychiatry Res 2001;104:213-20.
- Schmidtke K, Schorb A, Winkelmann G, Hohagen F. Cognitive frontal dysfunction in obsessive-compulsive disorder. Biol Psychiatry 1998;43:666-73.
- Basso MR, Bornstein RA, Carona F, Morton R. Depression accounts for executive function deficits in obsessive-compulsive disorder. Neuropsychiatry Neuropsychol Behav Neurol 2001;14:241-5.
- Moritz S, Birkner C, Kloss M, Jacobsen D, Fricke S, Böthern A, et al. Impact of comorbid depressive symptoms on neuropsychological performance in obsessive-compulsive disorder. J Abnorm Psychol 2001;110:653-7.
- Moritz S, Birkner C, Kloss M, Jahn H, Hand I, Haasen C, et al. Executive functioning in obsessive-compulsive disorder, unipolar depression, and schizophrenia. Arch Clin Neuropsychol 2002;17:477-83.
- Boone KB, Ananth J, Philpott L, Kaur A. Neuropsychological characteristics of non-depressed adults with obsessive-compulsive disorder. Neuropsychiatry Neuropsychol Behav Neurol 1991;4:96-109.
- Coetzer R, Stein DJ. Neuropsychological measures in women with obsessive-compulsive disorder and trichotillomania. Psychiatry Clin Neurosci 1999;53:413-5.
- Mataix-Cols D, Alonso P, Pifarré J, Menchón JM, Vallejo J. Neuropsychological performance in medicated vs. unmedicated patients with obsessive-compulsive disorder. Psychiatry Res 2002;109:255-64.
- Cummings JL. Obsessive-compulsive behavior in basal ganglia disorders. J Clin Psychiatry 1996;57:495-8.
- Deckersbach T, Otto MW, Savage CR, Baer L, Jenike MA. The relationship between semantic organization and memory in obsessive- compulsive disorder. Psychother Psychosom 2000;69:101-7.
- de Geus F, Denys DA, Sitskoorn MM, Westenberg HG. Attention and cognition in patients with obsessive-compulsive disorder. Psychiatry Clin Neurosci 2007;61:45-53.
- Savage CR, Baer L, Keuthen NJ, Brown HD, Rauch SL, Jenike MA. Organizational strategies mediate nonverbal memory impairment in obsessive-compulsive disorder. Biol Psychiatry 1999;45:905-16.
- Saxena S, Rauch SL. Functional neuroimaging and the neuroanatomy of obsessive-compulsive disorder. Psychiatr Clin North Am 2000;23:563-86.
- Freedman M. Object alternation and orbitofrontal system dysfunction in Alzheimer’s and Parkinson’s disease. Brain Cogn 1990;14:134-43.
- Freedman M, Oscar-Berman M. Bilateral frontal lobe disease and selective delayed response deficits in humans. Behav Neurosci 1986;100:337-42.
- Cavedini P, Ferri S, Scarone S, Bellodi L. Frontal lobe dysfunction in obsessive-compulsive disorder and major depression: a clinical- neuropsychological study. Psychiatry Res 1998;78:21-8.
- Moritz S, Kloss M, Jacobsen D, Kellner M, Andresen B, Fricke S, et al. Extent, profile and specificity of visuospatial impairment in obsessive- compulsive disorder (OCD). J Clin Exp Neuropsychol 2005;27:795- 814.
- Abbruzzese M, Ferri S, Scarone S. Wisconsin Card Sorting Test performance in obsessive-compulsive disorder: no evidence for involvement of dorsolateral prefrontal cortex. Psychiatry Res 1995;58:37-43.
- Kuelz AK, Hohagen F, Voderholzer U. Neuropsychological performance in obsessive-compulsive disorder: a critical review. Biol Psychol 2004;65:185-236.
- Veale DM, Sahakian BJ, Owen AM, Marks IM. Specific cognitive deficits in tests sensitive to frontal lobe dysfunction in obsessive- compulsive disorder. Psychol Med 1996;26:1261-9.
- Purcell R, Maruff P, Kyrios M, Pantelis C. Cognitive deficits in obsessive-compulsive disorder on tests of frontal-striatal function. Biol Psychiatry 1998;43:348-57.
- Grisham JR, Brown TA, Savage CR, Steketee G, Barlow DH. Neuropsychological impairment associated with compulsive hoarding. Behav Res Ther 2007;45:1471-83.
- Savage CR, Keuthen NJ, Jenike MA, Brown HD, Baer L, Kendrick AD, et al. Recall and recognition memory in obsessive-compulsive disorder. J Neuropsychiatry Clin Neurosci 1996;8:99-103.
- Abbruzzese M, Bellodi L, Ferri S, Scarone S. Frontal lobe dysfunction in schizophrenia and obsessive-compulsive disorder: a neuropsychological study. Brain Cogn 1995;27:202-12.
- Jurado MA, Junqué C, Vallejo J, Salgado P, Grafman J. Obsessive- compulsive disorder (OCD) patients are impaired in remembering temporal order and in judging their own performance. J Clin Exp Neuropsychol 2002;24:261-9.
- Nakao T, Nakagawa A, Nakatani E, Nabeyama M, Sanematsu H, Yoshiura T, et al. Working memory dysfunction in obsessive- compulsive disorder: a neuropsychological and functional MRI study. J Psychiatr Res 2009;43:784-91.
- Lecrubier Y, Sheehan DV, Weiller E, Amorim P, Bonora I, Harnett Sheehan K, et al. The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: reliability and validity according to the CIDI. Eur Psychiatry 1997;12:224-31.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). Washington, DC: American Psychiatric Press; 1994.
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56-62.
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR, et al. The Yale-Brown Obsessive Compulsive Scale. II. Validity. Arch Gen Psychiatry 1989;46:1012-6.
- Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin Card Sorting Test manual: revised and expanded. Odessa (FL), US: Psychological Assessment Resources Inc.; 1993.
- Reiten RM. Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills 1958;8:271-6.
- Pershad D. Construction and standardization of a clinical test of memory. Agra, India: National Psychological Corporation; 1977.
- Raven J, Raven JC, Court JH. Manual for Raven’s Progressive Matrices and Vocabulary Scales. Section 3, The Standard Progressive Matrices. Oxford, England: Oxford Psychologists Press / San Antonio, TX: The Psychological Corporation; 1998.
- Golden JC. Stroop Color and Word Test. Chicago (IL), US: Stoelting; 1978.
- Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol 1999;14:167-77.
- Krikorian R, Bartok J, Gay N. Tower of London procedure: a standard method and developmental data. J Clin Exp Neuropsychol 1994;16:840-50.
- Freedman M, Black S, Ebert P, Binns M. Orbitofrontal function, object alternation and perseveration. Cereb Cortex 1998;8:18-27.
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971;9:97-113.
- Tallis F. The neuropsychology of obsessive-compulsive disorder: a review and consideration of clinical implications. Br J Clin Psychol 1997;36:3-20.
- Rao NP, Arasappa R, Reddy NN, Venkatasubramanian G, Reddy YC. Emotional interference in obsessive-compulsive disorder: a neuropsychological study using optimized emotional Stroop test. Psychiatry Res 2010;180:99-104.
- Shin NY, Kang DH, Choi JS, Jung MH, Jang JH, Kwon JS. Do organizational strategies mediate nonverbal memory impairment in drug-naïve patients with obsessive-compulsive disorder? Neuropsychology 2010;24:527-33.
- Clayton IC, Richards JC, Edwards CJ. Selective attention in obsessive- compulsive disorder. J Abnorm Psychol 1999;108:171-5.
- Hwang SH, Kwon JS, Shin YW, Lee KJ, Kim YY, Kim MS. Neuropsychological profiles of patients with obsessive-compulsive disorder: early onset versus late onset. J Int Neuropsychol Soc 2007;13:30-7.
- Hermans D, Engelen U, Grouwels L, Joos E, Lemmens J, Pieters G. Cognitive confidence in obsessive-compulsive disorder: distrusting perception, attention and memory. Behav Res Ther 2008;46:98-113.
- Hashimoto N, Nakaaki S, Omori IM, Fujioi J, Noguchi Y, Murata Y, et al. Distinct neuropsychological profiles of three major symptom dimensions in obsessive-compulsive disorder. Psychiatry Res 2011;187:166-73.
- Roth RM, Milovan D, Baribeau J, O’Connor K. Neuropsychological functioning in early- and late-onset obsessive-compulsive disorder. J Neuropsychiatry Clin Neurosci 2005;17:208-13.
- Spitznagel MB, Suhr JA. Executive function deficits associated with symptoms of schizotypy and obsessive-compulsive disorder. Psychiatry Res 2002;110:151-63.
- Cabrera AR, McNally RJ, Savage CR. Missing the forest for the trees? Deficient memory for linguistic gist in obsessive-compulsive disorder. Psychol Med 2001;31:1089-94.
- Martinot JL, Allilaire JF, Mazoyer BM, Hantouche E, Huret JD, Legaut-Demare F, et al. Obsessive-compulsive disorder: a clinical, neuropsychological and positron emission tomography study. Acta Psychiatr Scand 1990;82:233-42.
- Jang JH, Kim HS, Ha TH, Shin NY, Kang DH, Choi JS, et al. Nonverbal memory and organizational dysfunctions are related with distinct symptom dimensions in obsessive-compulsive disorder. Psychiatry Res 2010;180:93-8.
- Moritz S, Kloss M, Jahn H, Schick M, Hand I. Impact of comorbid depressive symptoms on nonverbal memory and visuospatial performance in obsessive-compulsive disorder. Cogn Neuropsychiatry 2003;8:261-72.
- Segalàs C, Alonso P, Labad J, Jaurrieta N, Real E, Jiménez S, et al. Verbal and nonverbal memory processing in patients with obsessive-compulsive disorder: its relationship to clinical variables. Neuropsychology 2008;22:262-72.
- Trompenaars FJ, Masthoff ED, Van Heck GL, Hodiamont PP, De Vries J. Content validity, construct validity, and reliability of the WHOQOL- Bref in a population of Dutch adult psychiatric outpatients. Qual Life Res 2005;14:151-60.
- Trivedi JK, Dhyani M, Goel D, Sharma S, Singh AP, Sinha PK, et al. Neurocognitive dysfunction in patients with obsessive compulsive disorder. Afr J Psychiatry (Johannesbg) 2008;11:204-9.
- Rao NP, Reddy YC, Kumar KJ, Kandavel T, Chandrashekar CR. Are neuropsychological deficits trait markers in OCD? Prog Neuropsychopharmacol Biol Psychiatry 2008;32:1574-9.
- Kim MS, Jang KM, Kim BN. The neuropsychological profile of a subclinical obsessive-compulsive sample. J Int Neuropsychol Soc 2009;15:286-90.