East Asian Arch Psychiatry 2015;25:99-107
ORIGINAL ARTICLE
Dr Sandeep Grover, MD, Department of Psychiatry, Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh, India.
Dr Ajit Avasthi, MD, Department of Psychiatry, Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh, India.
Dr Adarsh Tripathi, MD, Department of Psychiatry, King George’s Medical University, Chowk, Lucknow, India.
Dr Andi J. Tanra, MD, Department of Psychiatry, Hasanuddin University Faculty of Medicine, Makassar, Sulawesi Selatan, Indonesia.
Dr Kok-Yoon Chee, MD, Department of Psychiatry and Mental Health, Kuala Lumpur Hospital, Kuala Lumpur, Malaysia.
Dr Yang-Lin He, MD, Department of Psychiatric Epidemiology, Shanghai Mental Health Center, Shanghai, PR China.
Prof. Helen F. K. Chiu, MD, Department of Psychiatry, The Chinese University of Hong Kong, Hong Kong SAR, PR China.
Dr Hironori Kuga, MD, Department of Neuropsychiatry, Kyushu University, Fukuoka, Japan.
Dr Min-Soo Lee, MD, PhD, Department of Psychiatry, College of Medicine, Korea University, Seoul, Korea.
Dr Mian-Yoon Chong, MD, PhD, Department of Psychiatry, Kaohsiung Chang Gung Memorial Hospital-Kaohsiung Medical Center and School of Medicine, Chang Gung University, Taiwan.
Dr Pichet Udormatn, MD, Department of Psychiatry, Faculty of Medicine, Prince of Songkla University, Songkhla, Thailand.
Dr Shigenobu Kanba, MD, PhD, Department of Neuropsychiatry, Kyushu University, Fukuoka, Japan.
Dr Shu-Yu Yang, PhD, Department of Pharmacy, Taipei City Hospital, Taipei, Taiwan.
Dr Tian-Mei Si, MD, PhD, Department of Psychiatry, Peking University Institute of Mental Health, Beijing, PR China.
Dr Kang Sim, MD, PhD, Institute of Mental Health, Buangkok View, Buangkok Green Medical Park Singapore, Taipei, Taiwan.
Dr Chay-Hoon Tan, MMed, PhD, Department of Pharmacology, National University of Singapore, Singapore.
Dr Winston W. Shen, MD, Department of Psychiatry, TMU-Wan Fang Medical Center, Taipei Medical University, Taipei, Taiwan.
Dr Yu-Tao Xiang, MD, PhD, Faculty of Health Sciences, University of Macau, Macao SAR, PR China.
Dr Norman Sartorius, MD, PhD, Association for the Improvement of Mental Health Programmes, Geneva, Switzerland.
Dr Naotaka Shinfuku, MD, PhD, Department of Psychiatry, Kobe University, Kobe, Japan.
Address for correspondence: Dr Sandeep Grover, Department of Psychiatry, Postgraduate Institute of Medical Education and Research, Chandigarh 160012, India.
Tel: (91-172) 2756807; Fax: (91-172) 2744401 / 2745078; Email: drsandeepg2002@yahoo.com
Submitted: 23 October 2014; Accepted: 18 November 2014
Abstract
Objective: To evaluate the prescription pattern of antidepressants in patients with medical co-morbidity from major psychiatric centres in Asia.
Methods: The Research on Asian Psychotropic Prescription Pattern for Antidepressants (REAP-AD 2013) collected data from 42 psychiatric centres in 10 Asian countries and regions. Antidepressant prescriptions of 2320 patients with various psychiatric disorders were evaluated. Of these, 370 patients who had specified medical co-morbidities formed the study cohort.
Results: Escitalopram (20%) and mirtazapine (20%) were the most commonly prescribed antidepressants in patients with medical co-morbidity followed by sertraline (16%), trazodone (15%), and paroxetine (12%). Overall, more than half (52%; 247/476) of prescriptions comprised selective serotonin reuptake inhibitors. Slightly less than two-thirds (63%; n = 233) of patients received at least 1 selective serotonin reuptake inhibitor. In addition, 79% of patients were prescribed only 1 antidepressant. The mean number of antidepressants used per patient was 1.25 (standard deviation, 0.56). There were subtle differences in the most preferred antidepressant across medical illnesses such as diabetes mellitus, liver dysfunction, acid peptic disease, and cerebrovascular disease. Differences were also seen in prescription patterns across different countries.
Conclusion: Although selective serotonin reuptake inhibitors formed the bulk of antidepressant prescriptions in the presence of medical co-morbidity, mirtazapine was also commonly used in the presence of medical co-morbidities. Specified medical morbidities do influence the selection of antidepressants.
Key words: Antidepressive agents; Asia; Comorbidity
Introduction
Depression is a common mental disorder and reported to have a high level of morbidity. Over the years research has confirmed the high prevalence rate of depression in patients with various medical illnesses. This high co-morbidity is now increasingly identified as a clinical and global health care issue.1 Presence of depression in medically ill patients is associated with a high level of disease burden, morbidity, mortality, poor medication and treatment compliance, higher health care costs, poor quality of life, as well as higher level of impairment.1,2 Accordingly adequate treatment of depression in medically ill patients is of paramount importance to improve treatment outcome of the primary medical illness.
Unfortunately, most drug trials that evaluate the efficacy and tolerability of antidepressants exclude patients with co-morbid medical illnesses. Although some recent studies have specifically evaluated the efficacy of certain antidepressants in the management of patients with various medical illnesses,3-6 most of them have been small. Due to the lack of adequate data from well-controlled trials, treatment guidelines often recommend avoidance of certain medications or reduced doses of antidepressants in the presence of certain medical illnesses. In general, the dictum is to start with the lower dose and use lower doses of antidepressants in the presence of medical illness. Further, the selection of medication is, more often than not, guided by tolerability profile and possible drug interactions.
Nonetheless there are a lack of data with respect to the real clinical use of antidepressants in patients with medical illnesses. Only few studies have evaluated the psychotropic prescription pattern in patients with medical illnesses, and that too in the hands of non-psychiatrists in hospitalised medically ill patients with co-morbid depression7 or elderly patients resident in long-term care facilities.8 Many studies have evaluated the antidepressant prescription patterns of psychiatrists and other health care professionals in the management of depression and other psychiatric disorders.9-15 Little is known about antidepressant prescription patterns of psychiatrists when a mentally ill patient has co-morbid medical illness. Therefore, this study attempted to examine the antidepressant prescription pattern in patients with various medical co-morbidities.
Methods
The data presented in this study emerged from the Research on Asian Psychotropic Prescription Pattern for Antidepressants (REAP-AD 2013), a pharmacoepidemiological study evaluating the antidepressant prescription pattern in patients with various mental disorders. The study centres were in Mainland China, Hong Kong, India, Indonesia, Japan, Korea, Malaysia, Singapore, Taiwan, and Thailand.
The study was carried out across 42 psychiatric centres in 10 countries and regions. Data collection followed the same procedure at each centre and was carried out between March and June 2013. Each centre recruited patients on a specified date. To be included in the study, patients were required to be prescribed an antidepressant on the day of survey irrespective of the primary psychiatric diagnosis. There were no exclusion criteria. On the day of the survey, all consecutive patients seen in the outpatient services, psychiatry inpatient services, and consultation- liaison services were eligible to participate in the study.
The study protocol was approved by the Ethics Review Committee of the institutes in which the study was conducted. All patients were informed about the aim of the study and only those who provided written or verbal consent according to the requirements of the relevant ethics committee were included. The diagnosis of specific psychiatric disorder was based on the ICD-1016 or DSM-IV criteria.17 As per the data collection, if a patient had co- morbid medical illness, it was also recorded. The study pro forma had provision for recording the following medical illnesses: myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, chronic pulmonary disease, rheumatic disease, peptic ulcer disease, mild liver disease, diabetes mellitus without complications, diabetes mellitus with complications, hemiplegia / paraplegia, renal disease, malignancy, moderate-to- severe liver disease, metastatic solid tumour, and acquired immunodeficiency syndrome / human immunodeficiency virus. Any other physical disorder was recorded as ‘other’ illnesses.
Data were analysed using the Statistical Package for the Social Sciences Windows version 14.0 (SPSS Inc., Chicago [IL], US). Data analysis included computation of frequency, percentage, mean, median, and standard deviation (SD).
Results
A total of 2320 patients with various psychiatric disorders were recruited as part of the REAP-AD 2013 study. Of these patients, 370 had at least 1 specified co-morbid medical illness, for example, diabetes mellitus or myocardial infarction. These patients formed the cohort for this study. Those who were diagnosed with at least 1 co-morbid physical illness other than the specified illnesses and thus coded as ‘other’ illnesses were excluded. The socio- demographic and clinical profiles of the study sample are shown in Table 1.
The mean (± SD) age of the study sample was 56.3 ± 16.3 years (range, 11-92 years). About one-third (n = 121; 32.7%) were aged > 65 years. Most patients were recruited from the university-affiliated general hospital psychiatric units and were outpatients. Also, 80% of patients had 1 psychiatric diagnosis and affective disorders (ICD-10 section F3) were the primary psychiatric diagnosis in two- thirds (65%). Overall 70% of patients had a diagnosis of affective disorder, i.e. either primary or co-morbid psychiatric disorder: 14% of patients had a primary psychiatric diagnosis of anxiety spectrum disorder, 62% had 1 physical co-morbidity, and 29% had 2 co-morbid physical illnesses. Among the specific type of primary physical illnesses (i.e. coded as the first co-morbid physical illness), diabetes mellitus (with or without complications) was recorded in about one-third of patients. Other common primary physical illnesses included cerebrovascular disease (12%), peptic ulcer disease (11%), liver disease (10%), and malignancy (7%). When multiple physical illnesses were taken into account, 40% of patients had diabetes mellitus, 14% had cerebrovascular disease, 12% had liver dysfunction and 12% had acid peptic disease.
Pattern of Antidepressant Prescription
As shown in Table 2, the most commonly prescribed antidepressants in patients with medical co-morbidity were escitalopram (20%), mirtazapine (20%), sertraline (16%), trazodone (15%), and paroxetine (12%), with respective mean dose of 14.5 mg/day, 25.4 mg/day, 70.8 mg/day, 61.7 mg/day, and 28.2 mg/day. Overall, more than half (52%; 247/476) of prescriptions comprised selective serotonin reuptake inhibitors (SSRIs). Slightly less than two-thirds (63%; n = 233) of patients received at least 1 SSRI. Four- fifths of patients were prescribed only 1 antidepressant; the mean number of antidepressants prescribed was 1.25 ± 0.56.
Further data analysis revealed that 61% of prescriptions of trazodone were in combination with another antidepressant. Other commonly prescribed antidepressants that were part of polypharmacy included paroxetine (39%), mirtazapine (38%), duloxetine (38%), escitalopram (34%), and venlafaxine (33%).
Use of Benzodiazepines
More than half (55%) of patients were also prescribed at least 1 benzodiazepine along with an antidepressant. The mean number of benzodiazepines prescribed to the study group was 0.74 ± 0.83.
Country Variation in Antidepressant Prescription Pattern
As shown in Table 3, there was wide variation in the most preferred antidepressant across different countries and regions. The polypharmacy rate also varied from 0% to 51.4%. Besides, SSRIs were used in at least two-thirds of patients in 6 of the 10 study countries and regions. The mean number of antidepressants prescribed varied from 1.0 to 1.75 and the mean number of benzodiazepines used varied from 0.16 to 1.42. The mean number of antidepressants prescribed was highest in Korea and that of benzodiazepines used was highest in Japan (Table 3).
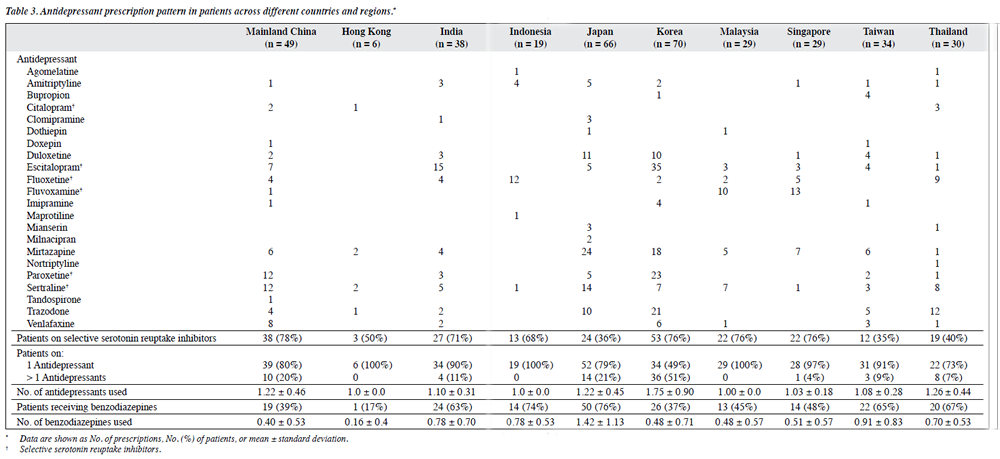
As shown in Table 4, in patients with diabetes mellitus, mirtazapine was the most commonly prescribed antidepressant, followed by sertraline, escitalopram, trazodone, fluoxetine, and paroxetine. Trazodone was the most commonly prescribed antidepressant in patients with liver dysfunction. Escitalopram was most commonly prescribed in patients with acid peptic disease, whereas for patients with cerebrovascular disease mirtazapine and sertraline were the most commonly prescribed antidepressants.
Escitalopram was the most commonly prescribed antidepressant in patients with depression, followed by mirtazapine, sertraline, trazodone, paroxetine, fluoxetine, and duloxetine. In terms of polypharmacy, the highest level was seen in patients with liver dysfunction: the mean number of antidepressants received by such patients was 1.39. A little more than half (50%-56%) of the patients with diabetes mellitus, liver dysfunction, acid peptic disease, and cerebrovascular disease were prescribed benzodiazepines with a mean number varying from 0.68 to 0.77 across different diagnostic groups.
Factors Associated with Prescription Patterns
There was no difference in prescription of any antidepressant between genders. Nonetheless those aged > 65 years were more frequently prescribed duloxetine (n = 18 vs. 14; Χ2 = –8.82; p = 0.003) and mirtazapine (n = 36 vs. 37; Χ2 = –11.4; p < 0.001), whereas those aged < 65 years were more often prescribed fluoxetine (n = 36 vs. 2; Χ2 with Yate’s correction = –13.13; p < 0.001).
We compared the antidepressant prescription pattern as per the primary psychiatric diagnosis: only patients with a primary diagnosis of mood (affective) disorders (F3) [n = 240] and neurotic, stress-related and somatoform disorders (F4) [n = 52] categories were included. Compared with those with F4 diagnosis, a higher proportion of patients with F3 diagnosis were prescribed escitalopram (n = 5 vs. 56; Χ2 = –4.86; p = 0.03). When compared with those with > 1 physical co-morbidity, those with only 1 physical co- morbidity were more frequently prescribed escitalopram (n = 61 vs. 12; Χ2 = –18.1; p < 0.001).
Discussion
Although many studies from different parts of the world including Asia have evaluated the prescription patterns of antidepressants in various settings,9-15 none have specifically focused on the antidepressant prescription pattern of psychiatrists for patients with a co-morbid physical illness. Thus, this study has attempted to examine the prescription pattern of antidepressants in patients with mental illnesses who also presented with certain specified physical co- morbidities. The prescription patterns obtained in this study possibly reflect how the presence of a co-morbid physical illness can influence the use of antidepressants. As no study has specifically evaluated the prescription patterns for antidepressants in the presence of medical morbidity, we evaluated the findings of this study against the prescription patterns reported in general and attempted to compare the findings with the recommendations of various treatment guidelines for management of depression per se18 or management of depression in adult patients with a chronic physical health problem.19
The findings of the present study revealed that SSRIs formed about half of the prescriptions of all antidepressants, serotonin-norepinephrine reuptake inhibitors formed 11.6% (55 out of 476) of all antidepressant prescriptions, and older antidepressants (trazodone, imipramine, clomipramine, amitriptyline, mianserin, doxepin and dothiepin) formed 19.1% (91 out of 476). Overall polypharmacy (i.e. concurrent use of 2 antidepressants) was seen in one-fifth of patients. Benzodiazepines were used in more than half of all patients. When we compared these findings with the existing studies from Asia and other parts of the world, this prescription pattern was very similar to that reported in studies that evaluated in general the prescription pattern for antidepressants or prescription given to patients with depression.9-15,20,21 Accordingly, it can be said that overall, and in the hands of psychiatrists, the prescription of various classes of antidepressants is not influenced by the presence of medical co-morbidity. Another way to interpret these findings is that newer antidepressants, especially SSRIs, are considered safe even in patients with medical co- morbidities by various treatment guidelines such as those of the American Psychiatric Association18 and National Institute for Health and Clinical Excellence.19
Comparison of the findings of this study with those of the previous REAP-AD 2003-2004 study10 that included patients from China, Korea, Japan, Singapore, and Taiwan revealed certain differences in terms of general prescription of individual antidepressants. In the previous study that evaluated antidepressant prescription in general, paroxetine was the most commonly prescribed antidepressant, followed by fluoxetine, trazodone, and fluvoxamine.10 A review of studies of prescription patterns in China reported melitracen / flupentixol combination, fluoxetine, and paroxetine to be the most commonly prescribed compounds.22 Some of the recent studies from India have reported escitalopram to be the most commonly prescribed antidepressant.20,21 In the present study, escitalopram and mirtazapine emerged as the most commonly used antidepressants. This difference in the commonly prescribed antidepressant can be interpreted as being influenced by the presence of medical co-morbidity or can be seen as a change in the prescription pattern of antidepressants in general over the years.
In terms of specific medical morbidity, mirtazapine was the most commonly used antidepressant in patients with diabetes mellitus. Other commonly used antidepressants in these patients were escitalopram, sertraline, trazodone, and fluoxetine. Some may consider the emergence of mirtazapine as one of the most commonly used antidepressants in diabetic patients as a cause of concern, because mirtazapine has been reported to be associated with weight gain and no randomised controlled trial has evaluated its efficacy in treatment of depression in patients with diabetes mellitus.23 Nonetheless a recent study did report the adverse effect of mirtazapine on blood glucose level in patients with diabetes mellitus, when used for a duration of 6 months. This controlled study from Korea evaluated the influence of mirtazapine on weight, glucose and haemoglobin A1c (HbA1c) levels, as well as lipid profile in patients with diabetes mellitus.24 Although mirtazapine was associated with weight gain compared with the control group, there was no significant difference between the 2 groups with respect to HbA1c level and lipid profile. In fact mirtazapine was reported to be associated with a reduction in HbA1c level and low-density lipoprotein level and increase in high-density lipoprotein level. One reason for higher use of mirtazapine in patients with diabetes mellitus in the present study was possibly its beneficial effect on sleep and pain, which might be a considerable problem in patients with diabetes mellitus.24
In patients with liver dysfunction, trazodone emerged as the most commonly prescribed antidepressant, followed by escitalopram, sertraline, mirtazapine, paroxetine, and fluoxetine. A significantly higher prescription of escitalopram and mirtazapine is understandable if used because of their minimal drug interactions at the hepatic metabolism. It is difficult to understand the higher use of trazodone, but it is quite possible that this was guided by sleep disturbance that is common in patients with hepatic dysfunction. This can be further understood by the fact that the mean dose of the trazodone (61.7 mg/day) used in the study was much less than the usual effective dose and was most commonly used (61%) as part of polypharmacy of antidepressants.
Escitalopram was the most commonly prescribed antidepressant in patients with acid peptic disease. Overall, SSRIs formed the bulk of the antidepressant prescriptions to patients with acid peptic disease. This finding was slightly surprising because in general SSRIs are associated with gastro-intestinal side-effects such as nausea, vomiting, and anorexia. There is also some concern about the risk of upper gastro-intestinal bleeding with SSRIs.25 Use of SSRIs in the presence of acid peptic disease may reflect the confidence of clinicians to use these medications irrespective of the presence of medical co-morbidity. Mirtazapine and sertraline were the most commonly prescribed antidepressants in patients with cerebrovascular disease. More frequent use of mirtazapine in such patients may have been influenced by the existing evidence that it is efficacious in management of post-stroke depression.26 A review of data also suggests that, despite the presence of heterogeneity in the trials evaluating the role of SSRIs in patients with stroke, SSRIs have been shown to improve anxiety and depression after stroke. Additionally, they have been shown to be associated with lower disability and neurological impairment.27
This study did not evaluate the first prescription to the patients. Hence, it is quite possible that in some cases antidepressants were prescribed before the diagnosis of physical illness was made. Therefore it cannot be concluded from this study that the prescription pattern was definitely influenced by the presence of physical illnesses. In the present study, the severity of psychiatric disorders as well as that of physical disorders was not evaluated. The severity of illnesses and complications associated with physical illnesses could have influenced the prescription pattern. This study also did not evaluate the specific reason for prescription of antidepressant and it is quite possible that in some cases antidepressants could have been prescribed for other benefits, for example to manage symptoms such as peripheral neuropathy, pain, and insomnia associated with physical disorders. In addition, this study did not evaluate the efficacy and tolerability of various antidepressants in patients with physical disorders. It is also likely that prescribed medications for physical illness could have influenced the choice of antidepressants. Such relationship was not studied. Future studies must attempt to overcome these limitations.
To conclude, the prescription pattern for antidepressants in patients with medical illnesses did not differ significantly to those without medical illnesses. Nonetheless when one looks at the specific medical illnesses, certain antidepressants are preferred to others. The types of antidepressants preferred are by-and-large supported by whatever little literature is available with regard to the efficacy of these agents in patients with various medical illnesses.
References
- Ramasubbu R, Beaulieu S, Taylor VH, Schaffer A, McIntyre RS; Canadian Network for Mood and Anxiety Treatments (CANMAT) Task Force. The CANMAT task force recommendations for the management of patients with mood disorders and comorbid medical conditions: diagnostic, assessment, and treatment principles. Ann Clin Psychiatry 2012;24:82-90.
- Baumeister H, Hutter N. Collaborative care for depression in medically ill patients. Curr Opin Psychiatry 2012;25:405-14.
- Rodin G, Lloyd N, Katz M, Green E, Mackay JA, Wong RK, et al. The treatment of depression in cancer patients: a systematic review. Support Care Cancer 2007;15:123-36.
- Ramamurthy G, Trejo E, Faraone SV. Depression treatment in patients with coronary artery disease: a systematic review. Prim Care Companion CNS Disord 2013;15. pii: PCC.13r01509.
- van der Feltz-Cornelis CM, Nuyen J, Stoop C, Chan J, Jacobson AM, Katon W, et al. Effect of interventions for major depressive disorder and significant depressive symptoms in patients with diabetes mellitus: a systematic review and meta-analysis. Gen Hosp Psychiatry 2010;32:380-95.
- Rayner L, Price A, Evans A, Valsraj K, Higginson IJ, Hotopf M. Antidepressants for depression in physically ill people. Cochrane Database Syst Rev 2010;(3):CD007503.
- Koenig HG, George LK, Meador KG. Use of antidepressants by nonpsychiatrists in the treatment of medically ill hospitalized depressed elderly patients. Am J Psychiatry 1997;154:1369-75.
- Conn DK, Goldman Z. Pattern of use of antidepressants in long-term care facilities for the elderly. J Geriatr Psychiatry Neurol 1992;5:228- 32.
- Sim K, Lee NB, Chua HC, Mahendran R, Fujii S, Yang SY, et al. Newer antidepressant drug use in East Asian psychiatric treatment settings: REAP (Research on East Asia Psychotropic Prescriptions) Study. Br J Clin Pharmacol 2007;63:431-7.
- Uchida N, Chong MY, Tan CH, Nagai H, Tanaka M, Lee MS, et al. International study on antidepressant prescription pattern at 20 teaching hospitals and major psychiatric institutions in East Asia: Analysis of 1898 cases from China, Japan, Korea, Singapore and Taiwan. Psychiatry Clin Neurosci 2007;61:522-8.
- Grover S, Kumar V, Avasthi A, Kulhara P. An audit of first prescription of new patients attending a psychiatry walk-in-clinic in north India. Indian J Pharmacol 2012;44:319-25.
- Bauer M, Monz BU, Montejo AL, Quail D, Dantchev N, Demyttenaere K, et al. Prescribing patterns of antidepressants in Europe: results from the Factors Influencing Depression Endpoints Research (FINDER) study. Eur Psychiatry 2008;23:66-73.
- Kjosavik SR, Hunskaar S, Aarsland D, Ruths S. Initial prescription of antipsychotics and antidepressants in general practice and specialist care in Norway. Acta Psychiatr Scand 2011;123:459-65.
- Nakao M, Takeuchi T, Yano E. Prescription of benzodiazepines and antidepressants to outpatients attending a Japanese university hospital. Int J Clin Pharmacol Ther 2007;45:30-5.
- Lako IM, Taxis K, Bruggeman R, Knegtering H, Burger H, Wiersma D, et al. The course of depressive symptoms and prescribing patterns of antidepressants in schizophrenia in a one-year follow-up study. Eur Psychiatry 2012;27:240-4.
- The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. Geneva: World Health Organization; 1992.
- Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Text Revision (DSM-IV). Washington, D.C.: American Psychiatric Association; 1994.
- Practice guideline for the treatment of patients with major depressive disorder. 3rd ed. Arlington, VA: American Psychiatric Association; 2010.
- National Institute for Health and Clinical Excellence. Depression in adults with a chronic physical health problem: treatment and management. 2009. (Clinical guideline 91). Available from: http://www.nice.org.uk/CG91. Accessed 18 Sep 2014.
- Grover S, Avasthi A, Kalita K, Dalal PK, Rao GP, Chadda RK, et al. IPS multicentric study: antidepressant prescription patterns. Indian J Psychiatry 2013;55:41-5.
- Grover S, Avasthi A, Sinha V, Lakdawala B, Bathla M, Sethi S, et al. Indian Psychiatric Society multicentric study: Prescription patterns of psychotropics in India. Indian J Psychiatry 2014;56;253-64.
- Zhang Y, Becker T, Kösters M. Preliminary study of patterns of medication use for depression treatment in China. Asia Pac Psychiatry 2013;5:231-6.
- Alam A, Voronovich Z, Carley JA. A review of therapeutic uses of mirtazapine in psychiatric and medical conditions. Prim Care Companion CNS Disord 2013;15. pii: PCC.13r01525.
- Song HR, Woo YS, Wang HR, Shim IH, Jun TY, Bahk WM. Does mirtazapine interfere with naturalistic diabetes treatment? J Clin Psychopharmacol 2014;34:588-94.
- Vidal X, Ibáñez L, Vendrell L, Conforti A, Laporte JR; Spanish- Italian Collaborative Group for the Epidemiology of Gastrointestinal Bleeding. Risk of upper gastrointestinal bleeding and the degree of serotonin reuptake inhibition by antidepressants: a case-control study. Drug Saf 2008;31:159-68.
- Niedermaier N, Bohrer E, Schulte K, Schlattmann P, Heuser I. Prevention and treatment of poststroke depression with mirtazapine in patients with acute stroke. J Clin Psychiatry 2004;65:1619-23.
- Mead GE, Hsieh CF, Lee R, Kutlubaev MA, Claxton A, Hankey GJ, et al. Selective serotonin reuptake inhibitors (SSRIs) for stroke recovery. Cochrane Database Syst Rev 2012;(11):CD009286.