Hong Kong J Psychiatry 2007;17:91-100
REVIEW ARTICLE
Dr Grace TY Leung, MBChB, MRCPsych (UK), Department of Psychiatry, Tai Po Hospital, New Territories, Hong Kong, China.
Prof Linda CW Lam, MRCPsych (UK), FHKCPsych, FHKAM (Psychiatry), Department of Psychiatry, The Chinese University of Hong Kong, New Territories, Hong Kong, China.
Address for correspondence: Dr Grace TY Leung, Department of Psychiatry, Tai Po Hospital, New Territories, Hong Kong, China.
Tel: (852) 2607 6111; Fax: (852) 2662 3568; E-mail:schroederleung@yahoo.com.hk
Submitted: 21 May 2007; Accepted: 4 July 2007
Abstract
Objectives: To selectively review longitudinal cohort studies examining the relationship of leisure activities to cognitive impairment in elders and to summarise proposed mechanisms of how such activities influence cognition.
Methods: Studies were identified in Ovid MEDLINE-R (2003 to March 2007) using a set of key words in titles: 'activities', 'activity', 'exercises', 'exercise', or 'leisure'. The results were cross-matched with another set of key words: 'cognitive', 'cognition', 'dementia', 'Alzheimer', 'Alzheimer's', or 'AD'. This search produced 376 studies. A total of 13 longitudinal cohort studies examining the relation between leisure activities and cognitive impairment in elders were found.
Results: Six of these studies focused on physical activities, one on cognitive activities, and the rest on general leisure activities. Cognitive activities showed more consistent beneficial effects on cognition, while the effects of physical and social activities were more inconsistent The mixed and inconsistent results might be attributed to differences in study designs, measurements, definitions and operational criteria of both participation in activities and outcome measures. Difficulties encountered in conducting these studies were demonstrated.
Conclusions: Further research is needed to elucidate the distinct effects of different activities on cognition, as well as to explore the necessary duration of such activity, evaluate the underlying mechanisms of benefit, and provide evidence upon which to base strategies to prevent cognitive impairment.
Key words: Cognition disorders; Dementia; Leisure activities; Motor activity
摘要
目的:選擇性回顧縱向定群研究,藉而檢視老人的休閒活動與認知障礙的關係,並總結這類活動是如何影響認知功能。
方法:在Ovid MEDLlNE-R資料庫內,使用以下關鍵字檢察2003年至2007年3月出版的研究:「活動」(' activities'及'activity')、「運動」 ('exercises’及'exercise')、「休閒」 ('Ieisure') ;再把所得結果用其他關鍵字交叉配對: 認知」('cognitive'及'cognition')、「痴呆」('dementia')、「阿氏痴呆症」 ('Alzheimer'、'Alzheimer's'及'AD'),得出376項研究。其中13項為探討老人休閒活動與認知障礙關係的縱向定群研究。
結果:13項研究中,有6項集中在體育活動, 1 項在認知活動,餘下的在一般休閒活動。研究結果顯示,不同的認知活動都比較一致地對認知有好處,而體育活動和社交活動的效果則相對參差。研究結果參差可能是因為研究設計、量度方法、對活動參與和結果衡量指標的定義和操作準則等各方面有所不同。這些研究亦談到進行研究時所遇到各種困難。
結論:如何解釋不同活動對認知所產生的截然不同的效果,尋找這類對認知有益的活動須進行多久,評估帶來這些益處的各種根本機制,為制訂防止出現認知障礙的策略提供事實根據,凡此種種都需要進一步研究。
關鍵詞:認知缺陷、痴呆、休閒活動、活動度
Introduction
Dementia is one of the devastating consequences of ageing, emerging as a major public health problem worldwide. Therefore, effective prevention strategies are increasingly appealing. Recent literature addressing the protective effects of participation in leisure activities on cognitive function has been accumulating. However, results from clinical studies are conflicting and difficult to compare. This is because the investigators are using different study designs, measurements, definitions, and operational criteria of both activity and cognitive measures in various populations. To illustrate the methodological considerations, recent longitudinal cohort studies examining these aspects in elderly people have been reviewed. Longitudinal cohort studies are of particular interest, as only a small number of randomised clinical trials are available and most are relatively short-term and small in scale. Mechanisms of how activities influence cognition are also discussed.
Methods
The Ovid MEDLINE-R (2003-March 2007) for original human research articles in English was searched using a set of key words in titles: ‘activities’, ‘activity’, ‘exercises’, ‘exercise’, or ‘leisure’. The results were cross-matched with another set of key words: ‘cognitive’, ‘cognition’, ‘dementia’, ‘Alzheimer’, ‘Alzheimer’s’, or ‘AD’. This search produced 376 studies. A total of 13 longitudinal cohort studies of elderly people regarding the association between leisure activities and cognition were found.
Results
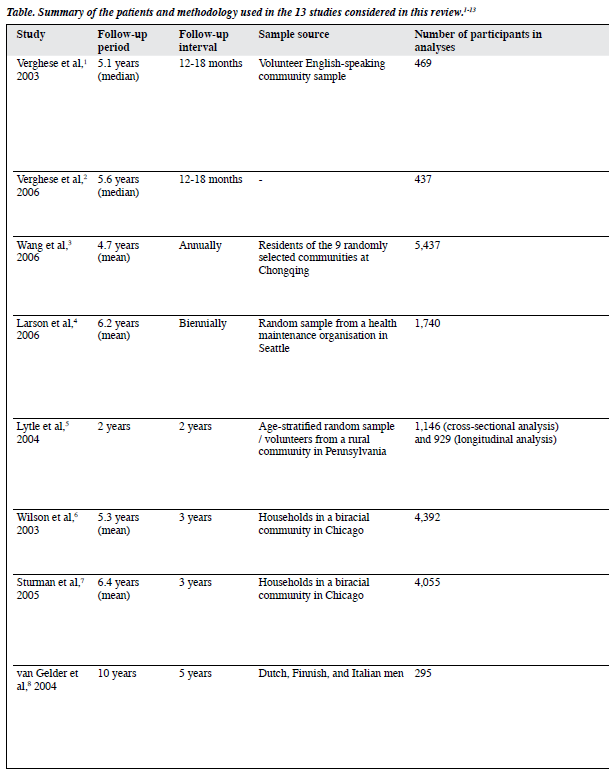
Most studies were developed from large-scale surveys, which also served other purposes. Six focused on physical activities, one on cognitive activities, and the rest on general leisure activities. Overall, results of cognitive activities showed a more consistent beneficial effect on cognition while the effects of physical and social activities were more inconsistent and equivocal. A summary of the methodology of the 13 studies evaluated is given in the Table.1-13
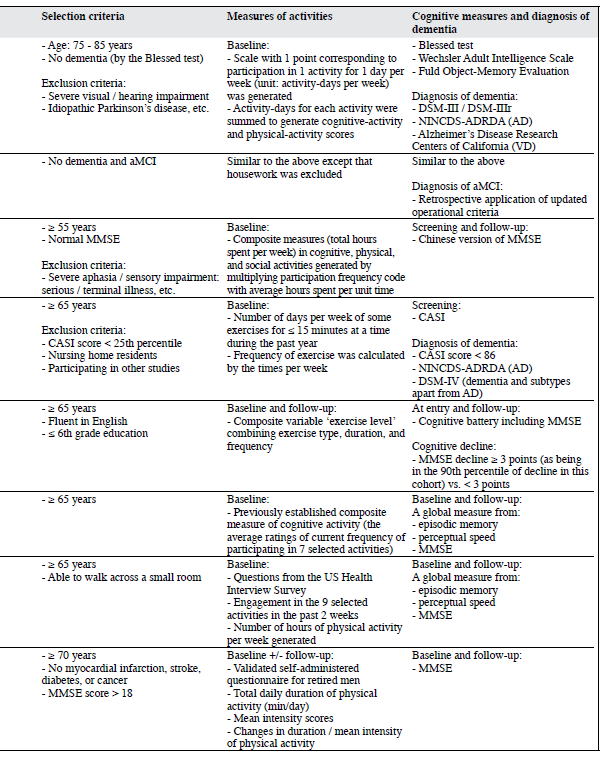
Verghese et al1,2 used data from the Bronx Aging Study to investigate the influence of leisure activities on the risk of developing dementia and amnestic forms of mild cognitive impairment (aMCI). At baseline, participation in cognitive and physical activities was queried, and cognitive- activity and physical-activity scores were generated based on the frequency of participation. Reading, playing board games, playing musical instruments, and dancing were associated with a lower risk of dementia.1 After adjustments for covariates like age, sex, education, baseline cognitive function, and the presence of medical problems, a 1-point increment in the cognitive-activity score was significantly associated with a reduced risk of dementia (hazard ratio [HR] = 0.93; 95% confidence interval [CI], 0.90-0.97)1 and aMCI (HR = 0.95; 95% CI, 0.91-0.99).2 The respective associations remained robust even after sequential exclusion of the subjects with possible preclinical dementia at baseline,1 and those who converted to dementia within 2 years of meeting the criteria for aMCI.2 Increased participation in cognitive activities was associated with a slower rate of cognitive decline in terms of episodic memory.1 Physical activities were not associated with reduced risk of dementia and aMCI.1,2
Wang et al3 investigated the association between leisure activities and cognitive impairment in a cohort of Chinese elderly people. Thirteen common leisure activities of Chinese urban elderly people were grouped into cognitive, physical, and social. Playing board games and reading were associated with a reduced risk of cognitive impairment, while watching television was associated with an increased risk. In the analysis of composite measures, only cognitive activities were associated with a reduced risk.
Larson et al4 explored whether regular physical exercise (defined as exercising at least 3 times a week) was associated with a reduced risk of dementia and Alzheimer’s disease (AD) using a sample selected from the Adult Changes in Thought study. Participants who exercised had a HR of 0.68 (CI, 0.48-0.96; p = 0.03) for developing dementia, compared to less frequent exercisers, even when potential confounders including the presence of apolipoprotein E genotype (APOE) e4 were adjusted for. The risk reduction was greater in those with lower levels of physical functioning at baseline. This finding suggests that exercise might reduce the risk of dementia through modulating the relationship between physical functioning and dementia.
Lytle et al5 used data from the MoVIES (the Monongahela Valley Independent Elders Survey) project to conduct a study of the relation between exercise, cognitive functioning, and cognitive decline. A composite variable ‘exercise level’ combining exercise type, duration, and frequency was generated within 3 levels: ‘high exercise’, ‘low exercise’, and the ‘no exercise’ reference group. High baseline exercise levels were associated with a reduced risk of cognitive decline 2 years later (odds ratio [OR] = 0.39; 95% CI, 0.19-0.78).
Wilson et al6 used data from the Chicago Health and Aging Project (CHAP) to examine the relation between cognitive activities and cognitive decline. Evidently, the frequency of cognitive activities was positively correlated with baseline cognitive function, and inversely associated with the rate of cognitive decline. A 1-point increase in cognitive-activity score was associated with approximately a 19% decrease in the annual rate of cognitive decline. Sturman et al7 conducted another study from the CHAP to examine the association between physical activities and cognitive decline after adjusting for participation in cognitive activities. When age, sex, race, and education were adjusted for, every additional hour of physical activity per week was associated with a slower rate of cognitive decline by 0.0007 U/year (p = 0.04). However, with further adjustments of participation in cognitive activities or factors like vascular illnesses and depression, or excluding participants having baseline global cognitive scores at or below the 10th percentile, the effects were no longer significant. The levels of physical activities reported were comparatively low, and the measure of frequency of participation in the past 2 weeks might not have adequately captured differences among subjects’ long-term participation in them.
Over a 10-year period, van Gelder et al8 investigated the independent association of duration and intensity of physical activities at baseline and change in these factors with change in cognitive functioning in elderly men. They used the data collected from the FINE Study (the Finland, Italy, and the Netherlands Elderly Study). Healthy subjects were recruited, since the cardiovascular system was generally fitter and might reflect a stronger contribution from physical activities. A decrease in duration of more than 60 min/day resulted in a cognitive decline of 2.6 times more than for those who maintained their exercise duration (p = 0.06). Men in the lowest intensity quartile at baseline had 1.8 to 3.5 times greater decline than those in other quartiles. A decrease in intensity of at least half a standard deviation was associated with a 3.6 times more decline than maintaining the level of exercise intensity (p = 0.003). There was no interaction between duration and intensity in relation to cognitive decline. A major limitation of the study was that the Mini-Mental State Examination constituted the only cognitive measure.
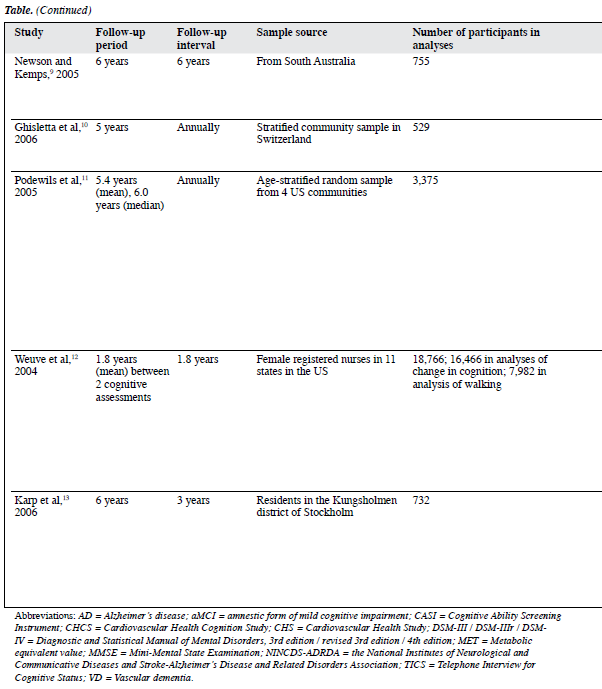
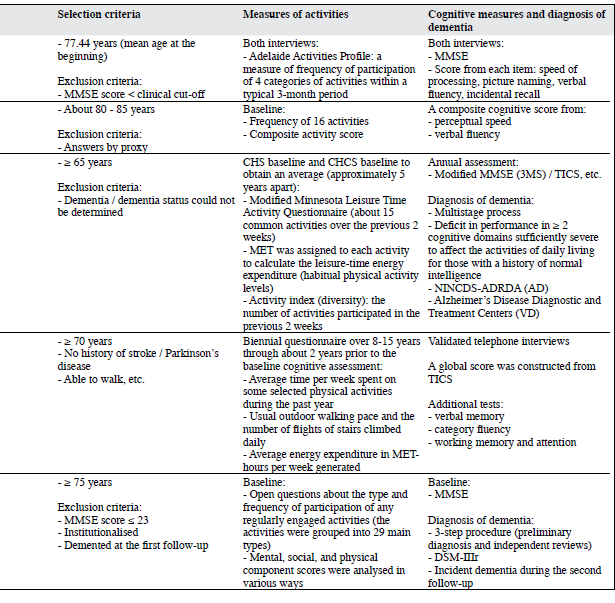
Studies from Newson and Kemps9 and Ghisletta et al10 are the only two studies that have considered the effect of sensory functioning on cognitive function. Using data from the Australian Longitudinal Study on Ageing, Newson and Kemps9 examined whether general lifestyle activities contributed to current cognition and cognitive change in elderly people. After sensory functioning (such as hearing and vision) was controlled for, such activity was a significant predictor of current processing speeds, picture naming, incidental recall, and verbal fluency, and of change in processing speed, picture naming, and incidental recall. The commonality analyses highlighted the importance of including measures of sensory functioning when examining the relations between age, activities, and cognition. The results also supported the proposal of activities as an important predictor of cognition. In a sample of very old individuals, Ghisletta et al10 investigated the relationship between activity engagement and performance in 2 cognitive domains (perceptual speed and verbal fluency tasks). They applied a longitudinal dynamic structural equation model to 5 repeated measurements of the Swiss Interdisciplinary Longitudinal Study on the Oldest Old. The results suggested that increased media and leisure activity engagement lessened decline in perceptual speed, but not in verbal fluency or performance, whereas cognitive performance did not affect change in activity engagement. The authors focused on the statistical procedures and only gave a very brief description of data collection.
Using data from an ancillary study (the Cardiovascular Health Cognition Study) of the Cardiovascular Health Study, Podewils et al11 examined how physical activities related to incident dementia and its subtypes, AD, and vascular dementia (VD). They showed that leisure-time energy expenditure was inversely associated with the risk of dementia, although after adjustments for covariates, the trend was no longer significant. The number of physical activities was inversely associated with the dementia risk, and this association persisted after multivariate adjustment. The multivariate-adjusted HR for all types of dementia comparing the highest with the lowest quartile of activity index yielded a value of 0.58 (95% CI, 0.41-0.83). The authors suggested 2 possible reasons. First, the reliability of the Minnesota Leisure Time Activity Questionnaire might be lower at low-to-moderate level of activities engaged by elderly people. Second, recall of number of activities might be more reliable than specifics like frequency and duration. Both factors are heavily weighted in calculating total energy expenditure. When analyses were stratified by APOE e4 carrier state, the inverse associations of energy expenditure and activity index with the dementia risk were limited to APOE e4 non-carriers; the associations were similar for AD and VD.
Weuve et al12 developed a study from the Nurses’ Health Study to examine the relation of long-term physical activities to cognitive function in a large cohort of old women. The analyses of physical activities were based on the average energy expenditures calculated from biennial questionnaires conducted over 8 to 15 years, terminating about 2 years prior to the baseline cognitive assessment. Analyses of walking were based on the assessment in 1986, through the questionnaire immediately preceding the baseline cognitive assessment. In examining walking, women who participated in vigorous exercises were excluded. Compared to women in the lowest physical activity quintile, a 20% lower risk of cognitive impairment for women in the highest quintile was observed (OR = 0.80; 95% CI, 0.67-0.95). Among women performing the equivalent of walking at an easy pace for at least 1.5 hours/week, the mean global cognitive scores were 0.06 and 0.07 units higher (equivalent to an age difference of 1.5 years) compared to walking of less than 40 min/week (p ≤ 0.03). Apart from the fluency category, higher levels of activities were associated with lesser declines in almost all the cognitive measures, and these trends were significant at the p < 0.01 level. However, a mean follow-up period of 1.8 years for measuring changes in cognitive function was relatively short, compared to other similar studies.
In the Kungsholmen Project, Karp et al13 aimed to verify the combined and individual effects of mental, social, and physical components of activities on the dementia risk. Three of the authors and 13 elderly raters scored the mental, social, and physical components in each of the 29 common leisure activities. When the components’ score sums were categorised into approximate quartiles, multi-adjusted relative risks (RRs) of dementia for subjects with higher mental, physical, and social components score sums were 0.71 (95% CI, 0.49-1.03), 0.61 (95% CI, 0.42-0.87), and 0.68 (95% CI, 0.47-0.99) respectively. The most beneficial effect was observed in subjects with high scores in two or more of the components (RR = 0.53; 95% CI, 0.36-0.78).
Discussion
Clinical studies of the association between leisure activities and cognition have only become popular in recent years. For the purpose of elucidating the methodological difficulties in conducting longitudinal cohort studies into this question, a search covering a relatively short period of time of one database seemed appropriate when this review was conducted. Some researchers repeated analyses of the same sample, focusing on slightly different research issues. Thus, the methods now applied are comparatively mature and well-developed, and an in-depth discussion based on them provides a foundation for further exploration and refinement.
There is growing evidence from biological studies that participation in activities has protective effects on cognitive decline, but mixed and inconsistent results have also been encountered in the 13 clinical research studies included in the Table. Aside from the limitations of longitudinal cohort studies, there are a multitude of problems.
First, the measurement of participation is difficult. Different theoretical definitions and operationalisations of activity engagement have been used. Whereas some studies dwell on general activities, others focus on specific domains. Studies that use composite measures often differ in exactly what their measures mean, how they are obtained, and how they are subsequently analysed. Moreover, most activities involve varying degrees of cognitive, physical, and social components, and it is unclear how to quantify the differences, particularly across diverse cultural and socio- economic backgrounds. Although Karp et al13 attempted to separate these components, and their methods had good inter-rater reliability, the components were not objectively measured. Many studies only assessed how often elderly people engaged in a restricted range of activities. These facilitated examination of how these activities related to cognition, but may also have underestimated their potential influence in general as they neglected variation among individuals, and activities other than those studied were omitted. Conversely, using a broader definition of an active lifestyle may examine engagement in wide-ranging activities integral to daily functioning, which can also influence cognition, and provides a more accurate reflection of the issues. Nowadays, this is important as a broad spectrum of daily activities is available to elders. Notably, the length of time retrospectively captured when measuring engagement is highly variable between studies.4,7,11,12 It is difficult to decide how long a period of measurement adequately captures differences among subjects’ long-term activity engagement. Other studies examine the relation of activities in early life to cognitive functioning in midlife14-16 and older age.17-20
Second, researchers have to disentangle the genuine influence of activities from the effects of preclinical dementia (i.e. reverse causation). The presence of preclinical dementia may reduce participation in activities and lead to subsequent overestimation of the protective effects. Resolution of this issue requires a long period of observation before the diagnosis of dementia, since in most types, there is a long period of cognitive decline preceding the diagnosis.21 Investigators usually tackle this problem by setting a higher threshold of baseline cognitive status,4 adjustment for baseline cognitive functioning in statistical analyses, and either procedurally13 or statistically excluding participants in whom dementia is diagnosed within a certain period of time after recruitment.
Third, inclusion of a reasonable list of covariates is crucial, in regard to assessments of activities and cognition. Indeed, additional information about participants may be a source of disagreement across investigations of the association. Among the 13 studies considered, only Newson and Kemps9 and Ghisletta et al10 documented sensory functioning as a covariate, and Podewils et al11 and Larson et al4 referred to it as the APOE e4 genotype. Changes in sensory functioning are important, as they influence the likelihood of engaging in activities, the functional capacity to perform activities, and the cognitive functioning of elderly people.22,23 Moreover, age-related changes in sensory functioning are thought to indicate age-based changes in the central nervous system, which are reflected in biological markers of ageing such as vision and hearing.24,25 Nevertheless, residual or unmeasured confounding is usually inevitable. For example, educational level and intellectual functioning may influence the choice of leisure activities, which are difficult to assess and may have differential effects on cognition.
The cognitive reserve hypothesis suggests that there is a developmental plasticity regarding cognition, whereby cognitive functioning of elderly people can be altered within the limits of the reserve capacity by environmental factors.26,27 Leading a more active lifestyle may result in functionally more efficient cognitive networks, thus providing a cognitive reserve that could delay the onset of the clinical manifestations of dementia.28 It has also been suggested that activation of nerve cells may prolong their optimal function throughout their life span.29
Various possible mechanisms as to how activities influence cognition have been proposed. Among these, the effect of participation in physical activities has been most widely studied. Participation in physical activities has been shown to have benefits in terms of cardiovascular diseases,30 stroke and white matter lesions,31,32 conditions implicated in the development of dementia. Benefits have also been proposed in patients who have hypertension,33 diabetes mellitus,34 and hypercholesterolaemia,35 all of which are related to cognitive decline. Physical activity may even promote endothelial nitric oxide production.36 Moreover, a relation between insulin and amyloid beta37,38 suggests that the benefits of aerobic activity on insulin resistance and glucose intolerance may be yet another mechanism by which physical activities can prevent or delay cognitive decline.39-41 Biological studies in humans and animals show that physical activity may improve cerebral blood flow, oxygen delivery,42 substrate exchange,43 and cerebral angiogenesis,44 whilst decreasing the accumulation of free radical oxidative proteins.45 Such activity also increases the expression of genes that promote neurogenesis and neuronal plasticity. These may release hormonal factors that facilitate neuronal creation and function (via brain-derived neurotrophic factor [BDNF], and epinephrine),46,47 and induce fibroblast growth factor in the hippocampus.48 The hippocampus is the area of the brain most susceptible to ischaemic damage, and one of the areas earliest to be affected by AD. It is suggested that a reduced loss of this part in the ageing brain is related to a higher level of physical fitness49; the level of BDNF in this part of the brain is diminished in patients with AD.50 Conceivably, physical activity increases the level of serotonin in the brain, thereby reducing stress and stress- induced hypercortisolaemia, stimulates regeneration of neurons within the hippocampus.51 In addition, involvement in physical activities facilitates learning, and is associated with mastery and self-efficacy.52 In turn, these attributes motivate persons to be more attentive to health needs and healthy behaviour.
The cognitive benefits derived in old age from cognitive and social activities are commonly explained by Schooler’s environmental complexity hypothesis.53 The enriched environment and cognitive complexity thus provided have been related to a variety of neuroplastic responses in adult animals, and include the formation of new neurons and synapses in brain regions that are critically involved in cognitive functioning.54,55 In humans, persistent engagement in cognitive activities may contribute to structural and functional reorganisation that makes active neural systems less vulnerable to disruption by AD pathology.56,57 Although some studies suggest that social networking and activities also contribute to a reduced dementia risk or attenuation of the rate of cognitive decline in elderly people,58,59 only a few mechanisms have been proposed in this regard. Social activities may affect the immune system,60 which in turn influences inflammatory processes in the brain that may be involved in dementia. Social networks may also promote overall health, particularly with respect to cardiovascular risk factors.
The relation between activity engagement and cognitive function is quite complex. Some longitudinal research studies suggest a reciprocal relationship, whereby cognitive function promotes activity engagement, which simultaneously enhances cognitive function.61,62 To elucidate the distinct effects of different activities on cognition, future research could benefit from analyses of specific activities, assessment of performance on a wider range of cognitive domains, and careful consideration of potential confounders. What period of life activity engagement is most relevant to preserving cognition and impacting the risk of dementia is also important. Further studies are needed to evaluate the underlying mechanisms. Longer-term randomised trials are deemed necessary to provide the evidence upon which prevention strategies for cognitive impairment can be based and for developing specific treatment programmes and recommendations for healthy ageing.
References
- Verghese J, Lipton RB, Katz MJ, Hall CB, Derby CA, Kuslansky G. Leisure activities and the risk of dementia in the elderly. N Engl J Med 2003;348:2508-16.
- Verghese J, LeValley A, Derby C, Kuslansky G, Katz M, Hall C, et al. Leisure activities and the risk of amnestic mild cognitive impairment in the elderly. Neurology 2006;66:821-7.
- Wang JY, Zhou DH, Li J, Zhang M, Deng J, Tang M, et al. Leisure activity and risk of cognitive impairment: the Chongqing aging study. Neurology 2006;66:911-3.
- Larson EB, Wang L, Bowen JD, McCormick WC, Teri L, Crane P, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med 2006;144:73-81.
- Lytle ME, Vander Bilt J, Pandav RS, Dodge HH, Ganguli M. Exercise level and cognitive decline: the MoVIES project. Alzheimer Dis Assoc Disord 2004;18:57-64.
- Wilson RS, Bennett DA, Bienias JL, Mendes de Leon CF, Morris MC, Evans DA. Cognitive activity and cognitive decline in a biracial community population. Neurology 2003;61:812-6.
- Sturman MT, Morris MC, Mendes de Leon CF, Bienias JL, Wilson RS, Evans DA. Physical activity, cognitive activity, and cognitive decline in a biracial community population. Arch Neurol 2005;62:1750-4.
- van Gelder BM, Tijhuis MA, Kalmijn S, Giampaoli S, Nissinen A, Kromhout D. Physical activity in relation to cognitive decline in elderly man: the FINE Study. Neurology 2004;63:2316-21.
- Newson RS, Kemps EB. General lifestyle activities as a predictor of current cognition and cognitive change in older adults: a cross- sectional and longitudinal examination. J Gerontol B Psychol Sci Soc Sci 2005;60:P113-20.
- Ghisletta P, Bickel JF, Lövdén M. Does activity engagement protect against cognitive decline in old age? Methodological and analytical considerations. J Gerontol B Psychol Sci Soc Sci 2006; 61:P253-61.
- 1 Podewils LJ, Guallar E, Kuller LH, Fried LP, Lopez OL, Carlson M, et al. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol 2005;161:639-51.
- Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F. Physical activity, including walking, and cognitive function in older women. JAMA 2004;292:1454-61.
- Karp A, Paillard-Borg S, Wang HX, Silverstein M, Winblad B, Fratiglioni L. Mental, physical and social components in leisure activities equally contribute to decrease dementia risk. Dement Geriatr Cogn Disord 2006;21:65-73.
- Richards M, Hardy R, Wadsworth ME. Does active leisure protect cognition? Evidence from a national birth cohort. Soc Sci Med 2003;56:785-92.
- Singh-Manoux A, Richards M, Marmot M. Leisure activities and cognitive function in middle age: evidence from the Whitehall II study. J Epidemiol Community Health 2003;57:907-13.
- Singh-Manoux A, Hilllsdon M, Brunner E, Marmot M. Effects of physical activity on cognitive functioning in middle age: evidence from the Whitehall II prospective cohort study. Am J Public Health 2005;95:2252-8.
- Fritsch T, Smyth KA, McClendon MJ, Ogrocki PK, Santillan C, Larsen JD, et al. Associations between dementia/mild cognitive impairment and cognitive performance and activity levels in youth. J Am Geriatr Soc 2005;53:1191-6.
- Dik M, Deeg DJ, Visser M, Jonker C. Early Life physical activity and cognition at old age. J Clin Exp Neuropsychol 2003;25:643-53.
- Crowe M, Andel R, Pedersen NL, Johansson B, Gatz M. Does participation in leisure activities lead to reduced risk of Alzheimer’s disease? A prospective study of Swedish twins. J Gerontol B Psychol Sci Soc Sci 2003;58:P249-55.
- Rovio S, Kåreholt I, Helkala EL, Viitanen M, Winbald B, Tuomilehto J, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurology 2005;4:705-11.
- Small BJ, Fratiglioni L, Viitanen M, Winbald B, Backman L. The course of cognitive impairment in preclinical Alzheimer disease: three- and 6-year follow-up of a population-based sample. Arch Neurol 2000;57:839-44.
- Anstey KJ, Smith GA. Interrelationships among biological markers of aging, health, activity, acculturation, and cognitive performance in late adulthood. Psychol Aging 1999;14:605-18.
- Spirduso WW. Physical dimensions of aging. Champaign IL: Human Kinetics Pub; 1995.
- Baltes PB, Linderberger U. Emergence of a powerful connection between sensory and cognitive functions across the adult lifespan: a new window to the study of cognitive ageing? Psychol Aging 1997;12:12-21.
- Hofer SM, Berg S, Era P. Evaluating the interdependence of aging- related changes in visual and auditory acuity, balance, and cognitive functioning. Psychol Aging 2003;18:285-305.
- Baltes PB, Reese HW, Lipsitt LP. Life-span developmental psychology. Annu Rev of Psychol 1980;31:65-110.
- Woodruff-Pak DS. Neural plasticity as a substrate for cognitive adaptation in adulthood and aging. In: Cerella J, Rybash J, Hoyer W, Commons ML, editors. Adult information processing: limits on loss. London: Academic Press; 1993:13-36.
- Scarmeas N, Stern Y. Cognitive reserve and lifestyle. J Clin Exp Neuropsychol 2003;25:625-33.
- Swaab DF. Brain aging and Alzheimer’s disease, “wear and tear” versus “use it or lose it”. Neurobiol Aging 1991;12:317-24.
- Wannamethee SG, Shaper AG. Physical activity in the prevention of cardiovascular disease: an epidemiological perspective. Sports Med 2001;31:101-14.
- Lee IM, Paffenbarger RS. Physical activity and stroke incidence: the Harvard Alumni Health Study. Stroke 1998;29:2049-54.
- Lee CD, Folsom AR, Blair SN. Physical activity and stroke risk: a meta-analysis. Stroke 2003;34:2475-81.
- Hagberg JM, Park JJ, Brown MD. The role of exercise training in the treatment of hypertension: an update. Sports Med 2000;30:193-206.
- Hu FB, Sigal RJ, Rich-Edwards JW, Colditz GA, Solomon CG, Willett WC, et al. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: a prospective study. JAMA 1999;282:1433- 9.
- Stefanick ML, Mackey S, Sheehan M, Ellsworth N, Haskell WL, Wood PD. Effects of diet and exercise in men and postmenopausal women with low levels of HDL cholesterol and high levels of LDL cholesterol. N Engl J Med 1998;339:12-20.
- Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, et al. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation 2000;101:2896-901.
- Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP, et al. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci U S A 2003;100:4162-7.
- Watson GS, Peskind ER, Asthana S, Purganan K, Wait C, Chapman D, et al. Insulin increases CSF Abeta42 levels in normal older adults. Neurology 2003;60:1899-903.
- Wareham NJ, Wong MY, Day NE. Glucose intolerance and physical inactivity: the relative importance of low habitual activity energy expenditure and cardiorespiratory fitness. Am J Epidemiol 2000;152:132-9.
- Thompson PD, Crouse SF, Goodpaster B, Kelley D, Moyna N, Pescatello L. The acute versus the chronic response to exercise. Med Sci Sports Exerc 2001;33(6 Suppl):S438-45,S452-3.
- Van Dam RM, Schuit AJ, Feskens EJ, Seidell JC, Kromhout D. Physical activity and glucose tolerance in elderly men: the Zutphen Elderly study. Med Sci Sports Exerc 2002;34:1132-6.
- Rogers RL, Meyer JS, Mortel KF. After reaching retirement age physical activity sustains cerebral perfusion and cognition. J Am Geriatr Soc 1990;38:123-8.
- Ide K, Secher NH. Cerebral blood flow and metabolism during exercise. Prog Neurobiol 2000;61:397-414.
- Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci U S A 1990;87:5568-72.
- Radak Z, Taylor AW, Ohno H, Goto S. Adaptation to exercise-induced oxidative stress: from muscle to brain. Exerc Immunol Rev 2001;7:90- 107.
- Cotman CW, Engesser-Cesar C. Exercise enhances and protects brain function. Exerc Sport Sci Rev 2002;30:75-9.
- Molteni R, Ying Z, Gómez-Pinilla F. Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. Eur J Neurosci 2002;16:1107-16.
- Gómez-Pinilla F, So V, Kesslak JP. Spatial learning and physical activity contribute to the induction of fibroblast growth factor: neural substrates for increased cognition associated with exercise. Neuroscience 1998;85:53-61.
- Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, et al. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci 2003;58:176-80.
- Phillips HS, Hains JM, Armanini M, Laramee GR, Johnson SA, Winslow JW. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer’s disease. Neuron 1991;7:695-702.
- Ball LJ, Birge SJ. Prevention of brain aging and dementia. Clin Geriatr Med 2002;18:485-503.
- American College of Sports Medicine Position Stand. Exercise and physical activity for older adults. Med Sci Sports Exerc 1998;30:992- 1008.
- Schooler C. Social structure effects and experimental situations: Mutual lessons of cognitive and social science. In: Schaie KW, Schooler C, editors. Social structure and aging: psychological processes. Hillsdale, NJ: Lawrence Erlbaum; 1989:129-47.
- Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long- term environmental enrichment. Ann Neurol 2002;52:135-43.
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature 2001;410:372-6.
- Wilson RS, Bennett DA. Cognitive activity and risk of Alzheimer’s disease. Curr Dir Psychol Sci 2003;12:87-91.
- Cummings JL, Vinters HV, Cole GM, Khachaturian ZS. Alzheimer’s disease: etiologies, pathophysiology, cognitive reserve, and treatment opportunities. Neurology 1998;51(1 Suppl 1):S2-17,S65-7.
- Seemen TE, Crimmins E. Social environment effects on health and aging: integrating epidemiologic and demographic approaches and perspectives. Ann N Y Acad Sci 2001;954:88-117.
- Fratiglioni L, Wang HX, Ericsson K, Maytan M, Winblad B. Influence of social network on occurrence of dementia: a community-based longitudinal study. Lancet 2000;355:1315-9.
- Seeman TE. Social ties and health: the benefits of social integration. Ann Epidemiol 1996;6:442-51.
- Schooler C, Mulatu MS, Oates G. The continuing effects of substantively complex work on the intellectual functioning of older workers. Psychol Aging 1999;14:483-506.
- Schooler C, Mulatu MS. The reciprocal effects of leisure time activities and intellectual functioning in older people: a longitudinal analysis. Psychol Aging 2001;16:466-82.