Hong Kong J Psychiatry 2008;18:28-35
REVIEW ARTICLE
Obesity and Alzheimer's Disease
肥胖症和阿爾茨海默病
CL Chang
鄭志樂
Dr CL Chang, MBBS, Department of Psychiatry, Queen Mary Hospital, Hong Kong, China.
Address for correspondence: Dr CL Chang, Department of Psychiatry, Queen
Mary Hospital, Hong Kong, China.
Tel: (852) 2855 4716; Fax: (852) 2819 3851; E-mail: changsja@yahoo.com.hk
Submitted: 17 July 2007; Accepted: 23 August 2007
Abstract
Objective:To review existing literature on obesity and Alzheimer's disease and evaluate evidence of an association between them.
Participants and Methods:A selected review of the epidemiologic studies published from 1987 to June '2007 from MEDLINE, EMBASE, PsychoINFO, and Cochrane Database about the relationship between obesity and Alzheimer's disease.
Results:There was some evidence that obesity may increase the risk of Alzheimer's disease; the association was stronger for midlife than late-life obesity.
Conclusion:Preliminary evidence indicates that obesity is a risk factor of Alzheimer's disease. Further large-scale, lifetime assessments are suggested to confirm the association and formulate effective measures to delay the onset of Alzheimer's disease.
Key words: Alzheimer disease; Dementia; Epidemiologic studies; Obesity; Risk factors
摘要
目的:分析精神分裂症患者使用抗精神病藥與肥胖症的關係。
參與者與方法:本橫斷面研究對象為根據<<心理疾病診斷統計手冊(第四版) >>診斷患有精神分裂症的病者;收集人口統計學資料與抗精神病相關的治療因素,包括使用抗精神病藥和伴隨藥物的類型和治療時間,以及有關人體數據,例如體重指數(BMI)和腰圍,並以世界衛生組織對亞裔人口BMI和腰圍的指引作出分類。
結果:63名(64.9% )和34名(35.1 %)患者各自接受非典型和典型的抗精神病藥治療。20名(20.6% )患者獲處方伴隨典型抗精神病藥。接受非典型抗精神病藥的一組,超重或肥胖症的現患率(BMI > 23.0 kg/m2) 為71 .4% (n = 45) ,而接受典型抗精神病藥的一組,現患率則為79.4% (n = 27)。腰圍較大與服用非典型抗精神病藥(p < 0.05) 和伴隨典型常規抗精神病長效注射藥(p < 0. 05) 呈正相關;以氯氮平治療的時間與患者腰圍呈負相關(r=0.006,P =0.004) ,但以奧氮平治療的時間與患者腰圍則呈正相關(r= 0.45, P = 0.03)。
結論:精神分裂症患者容易導致肥胖症。腰圍大與服用抗精神病藥物的類型和伴隨便用典型常規抗精神病長效注射藥有關。研究結果顯示,精神分裂症病人患有與肥胖症相關病症的風險較高。
關鍵詞:人體測量學、精神病藥劑、肥胖症、精神分裂症、重量增加
Introduction
In November 1906, Alois Alzheimer described the classical case of dementia as exhibiting typical clinical features with severe cognitive disturbances, delusions, and unpredictable behaviour. Neuropathological findings included military bodies (amyloid plaques) and dense bundles of fibrils (neurofibrillary tangles). In 1910, Kraepelin named this dementia as Alzheimer’s disease (AD).1 According to the Global Burden of Disease estimates for the 2003 World Health Report,2 dementia contributed 11.2% of years lived with disability in people aged 60 years and older. Alzheimer’s disease accounts for 50-60% of all patients with dementia, responsible for increasingly significant morbidity and mortality and ranked as the eighth leading cause of death among the elderly in the USA.3
Over the last century, we have gained more understanding about AD. The amyloid plaques and neurofibrillary tangles were found to develop as early as in the third decade.4 The need for a life course approach to understanding the causes of AD was recognised because the consequences and timing of AD are relevant throughout life. There is increasing evidence that vascular risk factors, such as hypertension,5-7 high cholesterol levels,7,8 and diabetes mellitus9,10 are also relevant, which often occur together with obesity. However, to date, there is no agreement on the association between obesity and AD.11,12 Postulated explanations for an association include the coincidence of these common disorders in the elderly, vascular and cerebrovascular diseases precipitating AD, an additive or synergistic pathogenesis of dementia, and misdiagnosis of vascular dementia and AD.
Obesity is a potentially remediable risk factor for many diseases, through lifestyle interventions.13 Moreover, numerous pharmacological and surgical treatments are also available.14-17 If obesity is a risk factor for AD, potentially it is modifiable. In this review, we examine the current literature and discuss the relationships between obesity and AD.
Obesity is a complex, multifactorial chronic health condition, characteristically giving rise to increased morbidity and mortality. The discovery of leptin and other hormones affecting fat has led to the concept that adipose tissue is an endocrine organ and not merely an energy storage depot. Body mass index (BMI) is currently the most common measure of general obesity, calculated as the body weight in kilograms divided by the square of body height in metres. In 1993, a WHO (World Health Organization) expert committee proposed BMI cut-off points of 25.0-29.9 kg/m2 for overweight grade 1, 30.0 to 39.9 kg/m2 for grade 2, and 40.0 kg/m2 or higher for grade 3.18 Body mass index also reflects the risk for type 2 diabetes and cardiovascular disease. However, the cut-off points are affected by age and ethnicity. Body mass index is less useful in body- builders who have high BMI due to their muscular builds rather than fat. Thus, other measures of obesity have been devised to overcome these limitations of BMI. The fact that abdominal adipose cells are more active in secreting the ‘fat hormones’ engendered the concept of ‘central obesity’. Common measures of central obesity include waist-to-hip ratio (WHR) and waist circumference (WC). A WHR ratio of greater than 0.9 in males and 0.8 in females was defined as central obesity. A WC of greater than 90 cm in men or 80 cm in women was defined as central obesity among Hong Kong population.19 Waist circumference has been shown to be better than BMI in assessing the risk of developing obesity-related co-morbidities.20
Alzheimer’s disease is more complicated than obesity, and to date, its aetiology is not fully understood. There is increasing consensus about a hypothesis which suggests that laying down of amyloid cascades in the central nervous system is the major mechanism.21 Beta-amyloid is derived from the amyloid precursor protein (APP) which is coded on chromosome 21. A mutation of APP gene appears to increase the production of β-amyloid, especially if such increased formation involved the 42 amino acid variant, the development of amyloid plaques was facilitated. Formation of neurofibrillary tangles, oxidation, glutamatergic excitotoxicity, inflammation, and activation of apoptotic cell death are considered secondary consequences of β-amyloid deposition. Finally, extensive neuronal death results in the clinical manifestation of dementia. Obesity is believed to increase the risk of AD through the amyloid cascade,22,23 leptin deficiency and the release of the β-secretase inhibition, as well as its association with dyslipidaemia and insulin resistance. Dyslipidaemia colocalises β- and γ- secretases and insulin resistance promotes the activity of γ- secretase.24-26 All of these processes facilitate the formation of the β-amyloid monomer. Several studies reported an association between APOE4 genotypes and obesity.23,27
APOE4 may act as a ‘pathological molecular chaperone’ that binds to soluble β-amyloid and enhances β-pleated sheet formation and amyloid fibril stability.27 Adipose tissue causes inflammation by secreting various cytokines, including interleukin-1, 2, and 6, resistin, tumour necrosis factor α, and C-reactive protein. These cytokines are thought to facilitate the formation of β-amyloid, which is also a proinflammatory agent. Exercise can increase the expression of anti-oxidant enzymes and reduce the expression of pro- oxidant enzymes.28,29 Fatty diets typically have an excess of saturated trans-fatty acids but are deficient in omega-3 fatty acids, fresh fruit and vegetables. Notably, trans-fatty acids increase oxidative stress and inflammation. In this literature review, we concentrate on epidemiological evidence of an association between obesity and AD. Its aims are: (1) to explore whether there is evidence to suggest that obesity is a risk factor for AD, and if so, (2) to identify possible differential effects of obesity at different ages on the risk of developing AD in later life.
Methods
MEDLINE (1966-2007), EMBASE (1980-2007), PsycINFO (1967-2007), and Cochrane Database of Systematic Reviews were searched. Studies published from 1987 to June 2007 were included. Literature about obesity was searched using the key words / terms ‘obesity’, ‘central obesity’, ‘general obesity’, ‘overweight’, ‘fat’, ‘adiposity’, ‘adiposity index’, ‘BMI”, ‘body mass index’, ‘waist to hip ratio’, ‘WHR’, ‘waist circumference’, ‘body anthropometry’, ‘bioelectric impedance’, and ‘skinfold thickness’. It yielded 705,438 references. Literature about AD was searched using the key words ‘Alzheimer’, ‘Alzheimer’s’ and ‘Alzheimer’s disease’. It yielded 113,870 references.
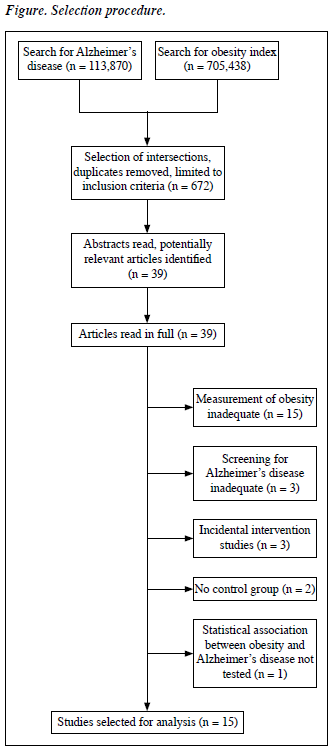
Potentially relevant articles were identified using the operator ‘AND’ to identify the intersection of the above 2 searches, and all duplicates removed. The search was further restricted to original work in the English language on human subjects — and yielded 672 articles. The abstracts of the latter were read to select original studies investigating the relationship between obesity and AD. In these studies, BMI, WHR, WC, skinfold thickness, and bioelectric impedance were accepted as measures of obesity. The diagnosis of AD was based on criteria detailed in the National Institute of Neurological and Communicative Disease and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA),30 the International Classification of Diseases (ICD), and the Diagnostic and Statistical Manual of Mental Disorders (DSM). Based on these criteria, 39 articles describing relevant original work were read in full. Among these, 15 articles not measuring any obesity index and 3 that did not use standard diagnostic criteria for AD were excluded. Similarly 3 studies investigating how various interventions can affect the risk of obesity in AD subjects, 2 others that had no control group, 1 that did not calculate the association between obesity and AD were also excluded. Finally, 15 papers fulfilled the selection criteria (Figure).
Results
To facilitate discussion from various perspectives, the studies were categorised into cross-sectional studies, prospective studies of midlife obesity, and prospective studies of late- life obesity.
Cross-sectional Studies
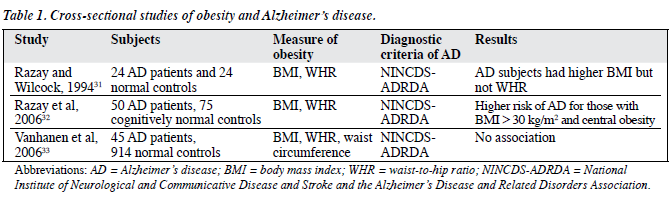
There were 3 cross-sectional studies on the relationship between obesity and AD (Table 1).31-33 In 1994, Razay and Wilcock31 conducted a study on 24 AD patients and 24 normal controls. The mean Mini-Mental State Score in AD patients was 12.4 and in controls it was 29.5, and both male and female AD subjects had higher BMIs than the controls; respective mean values in males and females being 25.8 kg/m2 and 25.5 kg/m2 versus 24.9 kg/m2 and 23.5 kg/m2. There was no significant difference between the groups for WHR for either sex. Razay et al32 conducted a similar study in 2006 with 50 AD patients and 75 cognitively normal controls; those with BMIs > 30 kg/m2 had about 9.5 times the risk of having AD (after adjustment for age, sex, and location). The odds ratio increased to 12.6 with further adjustments for systolic blood pressure, cholesterol and glucose values. Central obesity was defined as a WHR greater than 0.8 for women and greater than 0.9 for men. Those with central obesity had 2.5-fold the risk of having AD (after adjustment for age, sex, and location) and a further 2-fold risk after adjustment for systolic blood pressure, cholesterol and glucose. However, in another study with 914 normal controls and 45 AD patients, this association was not replicated by Vanhanen et al,33 though the ages of the subjects in both studies were comparable. Using WC cut-off values of > 102 cm in men and > 88 cm in women, in females 56.6% of normal controls and 62.5% of AD patients had abdominal obesity, compared to 23.8% of normal male controls and 30.8% of those with AD. However, this difference did not attain statistical significance. Neither did other obesity measures such as BMI (27.1 kg/m2 in normal controls and 27.5 kg/m2 in AD patients) and WHR (0.94 in normal controls and 0.95 in AD patients) yield any significant association.
Prospective Studies
In all, 12 prospective studies were identified and categorised according to the age of the subjects at recruitment.
Obesity in Midlife
There were 7 studies examining the association between obesity in midlife and AD (Table 2).34-40 Kalmijn et al34 and Stewart et al39 analysed the data from the Honolulu-Asia Aging Study using different approaches. Kalmijn et al34 investigated the relationship between BMI, and subscapular skinfold thickness and the incidence of AD in 3,734 men. Body mass index and subscapular skinfold thickness were measured in 1965, when the subjects were aged 45 to 68 years. Alzheimer’s disease was diagnosed according to NINCDS-ADRDA criteria between 1991 and 1993, at which time the subjects were aged 71 to 93 years. Neither BMI nor subscapular skinfold thickness were associated with AD. Since the major aim of the study was to investigate the relationship between metabolic syndrome and dementia, detailed results related to obesity and AD were not shown. Stewart et al39 studied the same subjects but extended the follow-up to 1999. Those who developed AD had mean midlife BMI values of 23.9 kg/m2 (SD = 3.0) while those without dementia also had mean values of 23.9 kg/m2 (SD = 2.7); compared to men without dementia, men with AD had an additional yearly weight loss of 0.3 kg (95% CI = 0.52- 0.08 kg) over the last 6 years.
In 2001 and 2005, Kivipelto et al35,37 studied subjects from the North Karelia Project and the FINMONICA study, respectively. Both studies together yielded 1,449 people aged 40 to 64 years in the original survey, who were followed up for about 21 years until they were aged 65 to 80 years, when they were re-examined. In the study in 2001,35 they found that the BMI of AD subjects (27.6 kg/m2, SD = 4.0) was slightly but significantly higher than that of the normal controls (26.5 kg/m2, SD = 3.7). In the study in 2005,37 they classified subjects into 3 groups according to BMI; values < 25 kg/m2 were considered normal, those with values of 25-30 kg/m2 were considered overweight, and those with higher values were considered obese. Alzheimer’s disease was encountered significantly more often among those with a higher midlife BMIs. Moreover, 6.9% of obese and 3.1% of overweight subjects were diagnosed as having AD, compared to 2.4% in the normal weight group.
Yamada et al36 studied 1,774 atomic bomb survivors and normal controls consisting of Hiroshima and Nagasaki residents. The aims of the study were to investigate the association between AD and vascular dementia and midlife risk factors. Neither BMI nor radiation dose appeared to have any significant effect on the prevalence of AD. Applying logistic regression with adjustment for age, sex and education, the odds ratio of each unit increase in BMI was 0.989 (p = 0.81), i.e. statistically non-significant. Rosengren et al38 conducted a study of BMI and the risk of dementia in 7,402 Swedish men, of whom 154 had a diagnosis of primary dementia and 22 were diagnosed as having AD. The lowest risk of dementia irrespective of cause was in those with BMIs in the range 20-22.49 kg/m2. For those men with higher BMIs, the relative risk increased linearly to 2.45 (95% CI, 1.17-5.12) in those with BMIs of ≥ 30.00 kg/m2. However, in the analysis in the subgroup with AD, the BMI was only slightly higher than in those without dementia and did not reach statistical significance. The BMI of AD patients and normal controls were 25.8 kg/m2 (SD = 3.2) and 25.5 kg/m2 (SD = 3.5), respectively.
The study conducted by Whitmer et al40 had the largest sample size and longest follow-up. They evaluated 10,136 participants of the Multiphasic Health Checkups (MHC) study between 1964 and 1973, when they reached the age of 40-45 years. The MHC examination included anthropometric measurements of height and weight. The BMI was calculated in kg/m2 and analysed according to the WHO criteria: underweight (< 18.5), normal weight (18.5-24.9), overweight (25.0-29.9), and obese (≥ 30). They also analysed BMI according to 6 more restricted kg/m2 categories: < 20, 20-22.49, 22.5-24.9, 25-27.49, 27.5-29.9, and ≥ 30, whilst also ascertaining those diagnosed with AD from January 1994 to June 2006 when the subjects were 65- 88 years old. These diagnoses were retrieved from medical records at Kaiser Permanente hospitals and clinic in visits to the neurology department, using the ICD-9 reference code. After adjustments for age, education, race, sex, marital status, smoking, hyperlipidaemia, hypertension, diabetes, ischaemic heart disease, and stroke, obese subjects (BMI > 30 kg/m2) had a 3.1-fold risk of AD (95% CI = 2.19-4.38). Overweight subjects (BMI = 25-30 kg/m2) had a 2.1-fold risk (hazard ratio = 2.09, 95% CI = 1.69-2.60).
Obesity in Late Life
There were 5 studies about late-life obesity and AD (Table 3).41-45
With the aim of determining risk factors for vascular dementia and AD in the general Japanese population, Yoshitake at el41 followed 828 non-demented residents of Hisayama Town aged 65 years or older for 7 years. Body mass index and skinfold thickness ratio were two of the parameters they measured. The respective mean BMI of men and women were 21.3 kg/m2 and 22.1 kg/m2. The relative risk of BMI was 0.75 (95% CI, 0.54-1.03), and that of skinfold thickness ratio was 0.85 (95% CI, 0.64-1.14); neither was found to be associated with AD.
Gustafson et al42 studied 70-year-olds and followed them up for 18 years. They interpreted the relationship between obesity and AD after adjustment for various factors including blood pressure, cardiovascular disease, cigarette smoking, socioeconomic status, and treatment of hypertension. Their female AD patients had significantly
higher BMIs than subjects with no dementia, when examined at the ages of 70, 75, and 79 years. At age 70, every 1.0 increment in BMI was associated with a 36% increase in AD risk. At ages 75 and 79 years, for every 1.0 increment in BMI the AD risk increased by 35% and 23% respectively. The association with obesity diminished as age increased. No such association was noted in men. Women with AD consistently had higher BMIs than normal subjects at ages 70, 75 and 79 years.
Buchman et al43 studied 832 Catholic clergies over 5.6 years. Contrary to findings from most studies, AD was associated with a low BMI at baseline. A person with a BMI of 21 kg/m2 at baseline had a more than 30% risk of developing AD than one with a BMI of 27 kg/m2. Each 1 unit less of BMI at baseline was associated with about 5% increment in the risk of AD. An annual decline of 1 BMI unit was associated with about 35% increase in the risk of AD compared to those who experienced no change in BMI. The Cache County study recruited 3,246 residents aged 65 or older44 and defined obesity as a BMI higher than 30 kg/m2. After 3.2 years of follow-up, obesity was associated with an increased risk of AD in females, with an adjusted hazard ratio of 2.23 (95% CI, 1.09-4.30). Adjustments were carried out for age, education, the APOE e4 allele, hypertension, high cholesterol levels, diabetes, stroke, history of coronary artery bypass graft surgery and myocardial infarction. However, in males the association was not statistically significant; the hazard ratio being 1.48 (95% CI, 0.41-4.18). Luchsinger et al45 followed up persons without dementia for 5 years. A total of 1,484 were available for anthropometric measures at first follow-up. Two hundred and fifty five persons were excluded because of prevalent dementia. Eight hundred and ninety three had available information about their BMI, and 907 had information available about their WC. The third BMI quartile (26.3-29.6) was found to be associated with a lower risk of AD compared to the first quartile (< 23.4). The fourth WC quartile was associated with a higher risk of AD in persons younger than 76 years, but not in those who aged 76 years or older.
Discussion
The results of these studies suggest that midlife obesity is likely to be a risk factor for AD. Whitmer et al’s study40 is particularly powerful, as it had a large sample and entailed a long follow-up duration. The negative results of other studies could be explained by under-diagnosis of AD, loss of participants and low levels of obesity. In Rosengren et al’s study,38 only 22 out of 176 patients with dementia were diagnosed as suffering from AD, which was below expectations. The Honolulu Heart Program39 initially recruited 4,768 participants but only 3,734 participants remained after screening for AD in 1991 to 1993.34
Moreover, the level of obesity in the Honolulu-Asia Aging Study was low and the mean midlife BMI was 23.9 kg/m2. These limitations should be appreciated while interpreting the results.
Studies on late-life obesity are also supportive of an association with AD.42 However, the interpretation of some findings is difficult due to methodological problems. In Buchman et al’s study,43 the mean age of those who developed AD (80 years) was significantly older than those who did not (74 years), in which case age rather than the BMI could account for the results. Moreover, the lifestyle and education of Catholic clergies probably differ from that of the general population. Studies show that education and physical activity appear to prevent AD.46,47 The duration of follow-up in Buchman et al’s study was relatively short.43 Interestingly, low BMI may actually be the result of unrecognised early AD; weight loss is reported to be common early in the course of AD.48-51 Luchsinger et al45 found that the third BMI quartile was associated with a lower risk of AD, though it did not mean that a high BMI was protective, as the fourth quartile did not experience a lower risk of AD. Short follow-up durations (up to 5 years) could cause considerable confusion in interpreting associations due to weight loss prior to the diagnosis of AD. On the other hand, ageing is characterised by loss of lean body mass and increased adipose tissue, but without weight gain. These changes may not be captured by BMI, which may therefore be less useful as an adiposity measure in elderly persons,52 and may be the reason Luchsinger et al45 also used WC, which yielded a positive association. For these reasons, WC has been proposed as a better adiposity measure than BMI in elderly persons.53-57
In conclusion, there is some evidence that obesity in mid and late life is a risk factor for AD. However, the evidence is based on research on western populations or American Japanese, making the results difficult to generalise for other ethnic groups. Language bias was inevitable, as this review only included studies published in English. Unpublished data were not identified due to limited resources. Most studies relied on BMI as a measure of obesity; very few measured central obesity. Thus, the relationship between AD and central obesity remains unclear and no conclusion on differences between general and central obesity can be made, nor were the underlying mechanisms linking adiposity to AD discussed. The heterogeneity of the studies makes it difficult to draw a single confident conclusion on the association between obesity and AD.
There are increasing evidences that the metabolic syndrome and its components are associated with AD.5-10,12 It is important to distinguish whether the apparent association between obesity and AD is a primary phenomenon or due to the confounding effects of other cardiovascular risk factors. Therefore, some of the above-mentioned studies reported results adjusted for various cardiovascular risk factors. Whitmer et al40 extensively adjusted for smoking, hyperlipidaemia, hypertension, diabetes, ischaemic heart disease, and stroke. Gustafson et al42 adjusted for blood pressure, cardiovascular disease, smoking, and treatment of hypertension. The Cache County study44 adjusted for hypertension, high cholesterol levels, diabetes, stroke, history of coronary artery bypass graft surgery and myocardial infarction. Associations encountered in studies using extensive adjustment for cardiovascular risk factors can therefore be regarded as relatively freer from the influence of corresponding confounding effects. However, prospective studies are prone to survival bias and small numbers of patients developing AD.
The association between obesity and AD implies that this risk may be potentially remediable. Sustained weight loss can be achieved by various methods ranging from lifestyle modification to more drastic interventions.58-61
Ideally, an equation calculating the total risk from the interaction of common risk factors should be developed. This might be accomplished in a way similar to the Boehringer- Mannheim Infarct Risk Spirit Calculator, which estimates the risk of myocardial infarction.61 Individuals should be stratified into different risk levels and different interventions recommended accordingly. In clinical practice, balancing psychological and social aspects and consideration of other risk factors is often more advantageous than achieving the optimal level of a single risk factor.
Finally, there are still many other areas in which the relationship between obesity and AD is not fully understood. Probably a life course approach in research is needed to fill these gaps. Nonetheless, it is well accepted that obesity is detrimental to physical health. As experience with lifestyle modification is safe, a balanced diet and physical exercise are therefore advocated for the sake of physical health and potential mental health.
Acknowledgements
I am greatly indebted to my supervisor, Dr KK Yu, for his invaluable advice. I would also like to express my gratitude towards the colleagues in Queen Mary Hospital. The assistance from the librarians of the Queen Mary Hospital and the University of Hong Kong in searching and obtaining replicas of all journal articles are also deeply appreciated.
Declaration
I declare that I have not received any financial support and have no conflicts of interest in preparing this literature review.
References
- Möller HJ, Graeber MB. The case described by Alois Alzheimer in 1911. Historical and conceptual perspectives based on the clinical record and neurohistological sections. Eur Arch Psychiatry Clin Neurosci 1998;248:111-22.
- World Health Report 2003 – Shaping the future. Geneva: World Health Organization; 2003.
- Centers of Disease Control and Prevention. Death: preliminary data for 2003. Natl Vital Stat Rep 2005;53:2.
- Finch CE. Developmental origins of aging in brain and blood vessels: an overview. Neurobiol Aging 2005;26:281-91.
- Skoog I, Lernfelt B, Landahl S, Palmertz B, Andreasson LA, Nilsson L, et al. 15-year longitudinal study of blood pressure and dementia. Lancet 1996;347:1141-5.
- Launer LJ, Ross GW, Petrovitch H, Masaki K, Foley D, White LR, et al. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging 2000;21;49-55.
- Kivipelto M, Helkala EL, Laakso MP, Hänninen T, Hallikainen M, Alhainen K, et al. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. BMJ 2001;322:1447-51.
- Kivipelto M, Helkala EL, Laakso MP, Hänninen T, Hallikainen M, Alhainen K, et al. Apolipoprotein E epsilon4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Ann Intern Med 2002;137:149-55.
- Launer LJ. Demonstrating the case that AD is a vascular disease: epidemiologic evidence. Ageing Res Rev 2002;1:61-77.
- Ott A. Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology 1999;53:1937-42.
- Skoog I. Status of risk factors for vascular dementia. Neuroepidemiology 1998;17:2-9.
- Skoog I. Vascular aspects in Alzheimer’s disease. J Neural Transm Suppl 2000;59:37-43.
- Wadden TA, Foster GD. Behavioral treatment of obesity. Med Clin North Am 2000;84:441-61.
- Mun EC, Blackburn GL, Matthews JB. Current status of medical and surgical therapy for obesity. Gastroenterology 2001;120:669-81.
- Ceruli J, Lomaestro BM, Malone M. Update on the pharmacotherapy of obesity. Ann Pharmacother 1998;32:88-102.
- Kolanowski J. A risk-benefit assessment of anti-obesity drugs. Drug Saf 1999;20:119-31.
- Orlistat for obesity. Med Lett Drugs Ther 1999;41:55-6.
- Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Consultation. WHO Technical Report Series Numbr 854. Geneva: World Health Organization; 1995.
- Ko GT, Cockram CS, Chow CC, Yeung V, Chan WB, So WY, et al. High prevalence of metabolic syndrome in Hong Kong Chinese — comparison of three diagnostic criteria. Diabetes Res Clin Pract 2005;69:160-8.
- Dobbelsteyn CJ, Joffres MR, MacLean DR, Flowerdew G. A comparative evaluation of waist circumference, waist-to-hip ratio and body mass index as indicators of cardiovascular risk factors. The Canadian Heart Health Surveys. Int J Obes Relat Metab Disord 2001;25:652-61.
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 2002;297:353-6. Erratum in: Science 2002;297:2209.
- Roher AE, Esh C, Rahman A, Kokjohn TA, Beach TG. Atherosclerosis of cerebral arteries in Alzheimer disease. Stroke 2004;35(11 Suppl 1): S2623-7.
- Sparks DL, Scheff SW, Liu H, Landers TM, Coyne CM, Hunsaker JC 3rd. Increased incidence of neurofibrillary tangles (NFT) in non- demented individuals with hypertension. J Neurol Sci 1995;131:162-9.
- Frears ER, Stephens DJ, Walters CE, Davies H, Austen BM. The role of cholesterol in the biosynthesis of beta-amyloid. Neuroreport 1999;10:1699-705.
- Simons M, Keller P, De Strooper B, Beyreuther K, Dotti CG, Simons K. Cholesterol depletion inhibits the generation of beta-amyloid in hippocampal neurons. Proc Natl Acad Sci USA 1998;95:6460-4.
- Kojro E, Gimpl G, Lammich S, Marz W, Fahrenholz F. Low cholesterol stimulates the nonamyloidogenic pathway by its effect on the alpha- secretase ADAM 10. Proc Natl Acad Sci USA 2001;98:5815-20.
- Nalbantoglu J, Gilfix BM, Bertrand P, Robitaille Y, Gauthier S, Rosenblatt DS, et al. Predictive value of apolipoprotein E genotyping in Alzheimer’s disease: results of an autopsy series and an analysis of several combined studies. Ann Neurol 1994;36:889-95.
- Berr C, Hauw JJ, Delaère P, Duyckaerts C, Amouyel P. Apolipoprotein E allele epsilon 4 is linked to increased deposition of the amyloid beta-peptide (A-beta) in cases with or without Alzheimer’s disease. Neurosci Lett 1994;178:221-4.
- Uusitupa MI, Karhunen L, Rissanen A, Franssila-Kallunki A, Niskanen L, Kervinen K, et al. Apolipoprotein E phenotype modifies metabolic and hemodynamic abnormalities related to central obesity in women. Am J Clin Nutr 1996;64:131-6.
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS- ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984;34:939-44.
- Razay G, Wilcock GK. Hyperinsulinaemia and Alzheimer’s disease. Age Ageing 1994:23:396-9.
- Razay G, Vreugdenhil A, Wilcock G. Obesity, abdominal obesity and Alzheimer disease. Dementia Geriatr Cogn Disord 2006;22:173-6.
- Vanhanen M, Koivisto K, Moilanen L, Helkala EL, Hänninen T, Soininen H, et al. Association of metabolic syndrome with Alzheimer disease. Neurology 2006;67:843-7.
- Kalmijn S, Foley D, White L, Burchfiel CM, Curb JD, Petrovitch H, et al. Metabolic cardiovascular syndrome and risk of dementia in Japanese-American elderly men. The Honolulu-Asia aging study. Arterioscler Thromb Vascu Biol 2000;20:2255-60.
- Kivipelto M, Helkala EL, Lasskso MP, Hänninen T, Hallikainen M, Alhainen K, et al. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. BMJ 2001;322:1447- 51.
- Yamada M, Kasagi F, Sasaki H, Masunari N, Mimiori Y, Suzuki G. Association between dementia and midlife risk factors: the Radiation Effects Research Foundation Adult Health Study. J Am Geriatr Soc 2003;51:410-4.
- Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kåreholt I, Winblad B, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol 2005;62:1556-60.
- Rosengren A, Skoog I, Gustafson D, Wilhelmsen L. Body mass index, other cardiovascular risk factors, and hospitalization for dementia. Arch Intern Med 2005;165:321-6.
- Stewart R, Masaki K, Xue QL, Peila R, Petrovitch H, White LR, et al. A 32-year prospective study of change in body weight and incident dementia: the Honolulu-Asia Aging Study. Arch Neurol 2005;62:55- 60.
- Whitmer RA, Gunderson EP, Quesenberry CP Jr, Zhou J, Yaffe K. Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Curr Alzheimer Res 2007;4:103-9.
- Yoshitake T, Kiyohara Y, Kato I, Ohmura T, Iwamoto H, Nakayama K, et al. Incidence and risk factors of vascular dementia and Alzheimer’s disease in a defined elderly Japanese population: the Hisayama Study. Neurology 1995;45:1161-8.
- Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch Intern Med 2003;163:1524-8.
- Buchman AS, Wilson RS, Bienias JL, Shah RC, Evans DA, Bennett DA. Change in body mass index and risk of incident Alzheimer disease. Neurology 2005;65:892-7.
- Hayden KM, Zandi PP, Lyketsos CG, Khachaturian AS, Bastian LA, Charoonruk G, et al. Vascular risk factors for incident Alzheimer disease and vascular dementia: the Cache County study. Alzheimer Dis Assoc Disord 2006;20:93-100.
- Luchsinger JA, Patel B, Tang MX, Schupf N, Mayeux R. Measures of adiposity and dementia risk in elderly persons. Arch Neurol 2007;64:392-8.
- Ritchie K, Lovestone S. The dementias. Lancet 2002;360:1759-66.
- Farlow MR. Alzheimer’s disease. Continuum Lifelong Learning Neurol 2007;13:39-68.
- White H, Pieper C, Schmader K, Fillenbaum G. Weight change in Alzheimer’s disease. J Am Geriatr Soc 1996;44:265-72.
- Cronin-Stubbs D, Beckett LA, Scherr PA, Field TS, Chown MJ, Pilgrim DM, et al. Weight loss in people with Alzheimer ’s disease: a prospective population based analysis. BMJ 1997;314:178-9.
- White H, Pieper C, Schmader K. The association of weight change in Alzheimer’s disease with severity of disease and mortality: a longitudinal analysis. J Am Geriatr Soc 1998;46:1223-7.
- Barrett-Connor E, Edelstein SL, Corey-Bloom J, Wiederholt WC. Weight loss precedes dementia in community-dwelling older adults. J Am Geriatr Soc 1996;44:1147-52.
- Stevens J, Cai J, Juhaeri, Thun MJ, Williamson DF, Wood JL. Consequences of the use of different measures of effect to determine the impact of age on the association between obesity and mortality. Am J Epidemiol 1999;150:399-407.
- Visscher TL, Seidell JC, Molarius A, van der Kuip D, Hofman A, Witteman JC. A comparison of body mass index, waist-hip ratio and waist circumference as predictors of all-cause mortality among the elderly: the Rotterdam study. Int J Obes Relat Metab Disord 2001;25:1730-5.
- Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr 2004;79:379-84.
- Going S, Williams D, Lohman T. Aging and body composition: biological changes and methodological issues. Exerc Sport Sci Rev 1995;23:411-58.
- Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in adults — The Evidence Report. National Institutes of Health. Obes Res 1998;6(Suppl 2):S51-209.
- Wadden TA, Foster GD. Behavioral treatment of obesity. Med Clin North Am 2000;84:441-61.
- McGuire MT, Wing RR, Klem ML, Hill JO. Behavioral strategies of individuals who have maintained long-term weight losses. Obes Res 1999;7:334-41.
- Methods for voluntary weight loss and control. NIH Technology Assessment Conference Panel. Consensus Development Conference, 30 March to 1 April 1992. Ann Intern Med 1993;119:764-70.
- Assmann G, Schulte H. Results and conclusions of the Prospective Cardiovascular Munster (PROCAM) Study. In: Assmann G, editor. Lipid metabolism disorders and coronary heart disease. Munchen: MMV Medizin Verlag; 1989.
- Assmann G, Schulte H. Modelling the Helsinki Heart Study by means of risk equations obtained from the PROCAM Study and the Framingham Heart Study. Drug 1990;40(Suppl 1):S13-8.