Hong Kong J Psychiatry 2008;18:107-14
ORIGINAL ARTICLE
尼日利亞伊巴丹一所大學醫院內癌症病人抑鬱症的現患率及預測因子
Dr Folorunsho T Nuhu, MBBS, FMCPsych, Department of Psychiatry, University College Hospital, Ibadan, Nigeria.
Dr Olabisi A Odejide, MBBS, FRCPsych, FMCPsych, FWACP, Department of Psychiatry, University College Hospital, Ibadan, Nigeria.
Dr Kazeem O Adebayo, MBBS, FWACP, Department of Psychiatry, University College Hospital, Ibadan, Nigeria.
Dr Olurotimi Adejumo, MBBS, Department of Psychiatry, University College Hospital, Ibadan, Nigeria.
Address for correspondence: Dr FT Nuhu, Department of Psychiatry, University College Hospital, Ibadan, Nigeria.
Tel: (2348) 056073975; Fax: (2348) 060465845; E-mail: funshonuhu@yahoo.com
Submitted: 28 February 2008; Accepted: 19 June 2008
Abstract
Objective: To determine the prevalence of depression in cancer patients and the factors that predict its occurrence.
Participants and Methods: Two hundred and ten consecutive patients admitted into the radiotherapy and affiliated wards in the University College Hospital, Ibadan who had cancers of the breast, cervix, colon / rectum or prostate were interviewed using the socio-demographic and clinical questionnaire, the Structured Clinical Interview for DSM-N diagnosis, and the World Health Organization Quality of life questionnaire.
Results: The sample consisted of63 (30%) males and 147 (70%) females. Sixty eight (32%) of the subjects had breast cancer, 59 (28%) bad cervical cancer. 40 (19%) had colo-rectal cancer. while the remaining 43 (21%) had prostate cancer. The prevalence of depression was found to be 30%. Having an advanced stage of cancer, presence of pains, and having a family history of mental illness were significantly associated with being depressed. Depressed patients had poorer quality of life.
Conclusion: Depression is common among cancer patients and has a negative effect on quality of life. Those at risk have advanced stages of cancer, pains, and a family history of mental illness. Special attention should be paid to these categories of cancer patients.
Key words: Depression; Neoplasma; Pain; Prevalence; Quality of life
摘要
目的:探討癌症病人抑鬱症的現患率及預測因子。
參與者與方法:用社會人口學及臨床問卷( DSM-IV診斷) 及世界衛生組織的「生活質素問卷」,訪問連績210位入住尼日利亞伊巴丹一所大學醫院內的放射治療及相關病房內,患有乳癌、子宮頸癌、大腸癌、直腸癌, 或前列腺癌的病人。
結果:受訪者共210名,包括63名(30% ) 男性和147名( 70%) 女性; 其中68名( 32% ) 患有乳癌、59名( 28% ) 患子宮頸癌、40名( 19%) 有大腸/直腸癌,其餘的43名( 21 % ) 有前列腺癌。抑鬱症的現患率為30% 。以下因素皆與抑鬱症顯著相關: 末期癌症、痛症,以及家族有精神病病史。抑鬱症病人生活質素亦較差。
結論:抑鬱症常見於癌症病人,並對病人產生負面影響。末期癌症、痛症,以及家族有精神病 病史均為高風險因素。因此必須對這類病人多加注意。
關鍵詞:抑鬱症、腫瘤、疼痛、現患率、生活質素
Introduction
Cancer, a colloquial expression for malignant neoplasm, is associated with significantly high morbidity and mortality and its diagnosis is often regarded by many as a death sentence.1 Some cancer patients experience physical symptoms such as pain, weight loss, ulceration at the cancer site, swelling, bleeding, and impaired sexual functioning.2 These patients also exhibit psychological symptoms of anxiety disorder, adjustment disorder, post-traumatic stress disorder, depression, and organic mental illness.3,4
Psychiatric disorders are common in cancer patients but are often unrecognised by their oncologists. Craig and Abeloff5 reported that failure of health care providers to recognise psychiatric symptoms in cancer patients may be due to their shying away from probing into psychological symptoms that are not disclosed.
Previous studies in cancer populations have reported the prevalence of depression as low as 9%6 and as high as 45%.7 Some of the studies considered depressive symptoms rather than depressive disorder; some were carried out among severely ill patients, while in others the sample size was too small. Only a few of such studies have been conducted in Nigeria.7
Patients at highest risk for depression are those with a history of affective disorder or alcoholism, advanced stages of cancer, poorly controlled pain and treatment with medication or concurrent illnesses associated with depressive symptoms.8
Case examination of depressed cancer patients suggests that illness and treatment factors, even though important, may not be the primary risk factors for psychological morbidity. External stressors such as poor family support and past sexual abuse (compounded by illness and treatment factors) may be more relevant in adolescents.6 Grassi and Rosti9 reported that awareness of the diagnosis of cancer, history of previous psychiatric disorders, pain and stress factors are predictors of depression in cancer patients. Using a logistic regression analysis, Kugaya et al10 concluded that having an advanced stage of cancer and living alone were significantly associated with having psychological distress. Several studies have shown that cancer patients with co- morbid depression are more likely to have a poor quality of life compared to those who are not depressed.11-14
There is limited information on the prevalence of psychiatric disorders among cancer patients in Nigeria and specifically there is paucity of data on the factors associated with the development of depression in this population of patients. It is against this background that cancer patients in Ibadan were studied to determine the prevalence and predictors of depression and the effects of depression on their quality of life.
Methods
This was part of a larger study examining depression and quality of life among cancer patients on treatment, conducted in the radiotherapy, gynaecology and surgical wards of the University College Hospital Ibadan, Nigeria. It is an 812-bed teaching hospital located in Ibadan North local government area of Oyo State in South Western Nigeria, and is a referral centre for other hospitals and clinics in Oyo and neighbouring states.
Subjects
The sample consisted of all cancer patients consecutively admitted into the radiotherapy, gynaecology and surgical wards between May and November 2006 who met the entry criteria.
The inclusion criteria were: a histological diagnosis of cancer of the cervix, breast, prostate or colon / rectum; aged 18 years and above. Patients with a history of chronic mental illness, e.g. schizophrenia, pre-existing organic brain pathology before the onset of cancer such as dementia or mental retardation, acute confusional state (i.e. delirium) during interview, and chronic physical illness (such as cardiac, renal, hepatic, cerebrovascular, pulmonary or infectious disease) were excluded. Three patients refused consent because they felt they were in the hospital for treatment and not research were also excluded.
Procedure
Ethical approval was obtained from the University of Ibadan / University College Hospital Institution Review Committee (UI / UCH IRC). Subjects were interviewed using the socio-demographic and clinical questionnaire, Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) to make a diagnosis of depression, the Montgomery-Asberg Depression Rating Scale (MADRS) to determine the severity of depression, and the World Health Organization Quality of Life questionnaire (WHOQOL- Bref) to measure quality of life. The socio-demographic and clinical questionnaire was designed by one of the authors (FTN). It addressed items such as age, gender, previous and family history of mental illness, use of alcohol, living status, and source of support (financial and psychosocial). Other items in the questionnaire asked about type and stage of cancer, duration of cancer symptoms, type of treatment, physical manifestations of the cancer such as swelling and ulceration, and the presence of pain. Subjects who reported that they had pain were asked to rate the pain from very mild, mild, moderate, severe to very severe (similar to the Verbal Rating System) and to indicate the site. We checked patients’ case notes to confirm that the information given by the patients including the analgesic drugs prescribed by their clinicians (in cases with pain) as well as the family and social history (including the use of alcohol) obtained from patients’ relatives by the clinicians and nurses. Two of the authors (FTN and KOA) attended a 5-day training programme in the use of SCID organised for participants in the World Mental Health Project, 2000 and interviewed all subjects in this study.
The SCID has been used in a comprehensive epidemiological survey of mental health in Nigeria (World Mental Health Project, 2000), part of which was presented at the annual conference of the Association of Psychiatrist in Nigeria, Calabar, 2003 (Gureje and Adebayo). The instrument has been shown to have good reliability for categorical constructs of DSM-IV diagnoses,15,16 and good standard validity.17,18
The MADRS is a scale that assesses the severity of depression. The observer-rated MADRS19 is a 20-item scale that measures the severity of depressive symptoms. While the scale correlates well with the Hamilton Depression Rating Scale,20 its lack of emphasis on physical symptoms has led some investigators to suggest that it is a more valid measure of depression severity than the Hamilton Depression Rating Scale.21 Cut-off scores for the MADRS were as follows: 0-6 (normal), 7-19 (mild), 20-34 (moderate), and > 34 (severe).22
We administered the short version of the WHOQOL- Bref to measure the quality of life of the patients.23 We compared the quality of life of depressed and non-depressed cancer patients. All the instruments were translated from English to ‘Yoruba’ (the local language of the people in the study area) through the process of back-translation.24 The Yoruba version was administered to patients who could not understand English. Other essential information such as the presence of a concurrent physical illness was obtained from patients’ case notes. The clinicians managing the patients were contacted, whenever there was any doubt. All the patients who took part in the study gave informed consent.
Data Analysis
The 11th edition of the Statistical Package for the Social Sciences (SPSS-11) software was used for analysis. Descriptive statistics were calculated for all variables, which included means and standard deviation. Student’s t test was used to compare the mean values for quality of life. Frequency distribution and cross tabulations were generated and the Chi-square (X2) test was used to compare proportions and also to investigate associations between categorical variables. All variables found to be statistically significant in the bivariate analysis were entered into a multiple logistic regression analysis to identify the predictors of depression. A P value of < 0.05 was taken as statistical significance.
Results
Socio-demographic Characteristics of the Subjects
The socio-demographic characteristics of the subjects are shown in Table 1. A total of 210 were interviewed. Of these, 63 (30%) were males while 147 (70%) were females. The mean age of all the subjects was 53 (standard deviation [SD], 4) years; the mean age for males was 60 (SD, 6) years while for females it was 50 (SD, 5) years. Eight (4%) of the patients were within the age-group of ‘21-30’ years, 112 (53%) were aged 41 to 60 years, while 1 was older than 80 years. The majority of subjects (185, 88%) reported that they lived with either their spouses or children or both; only 6 (3%) subjects lived alone. One hundred and nineteen (57%) could not say how much they earned per month before the onset of their illness, 29 (14%) earned less than US$84 in a month, while 4 (2%) earned more than US$420 per month. Forty eight (23%) subjects had no formal education, 38 (18%) had completed primary education, 35 (17%) had completed secondary education, and 56 (27%) had tertiary education.
One patient did not receive support for the illness from anybody, while the majority, 204 (97%) received support (psychosocial and financial) from their relatives. Only 4 (2%) received financial support from government.
Clinical Characteristics of the Subjects
About a half (50%) of the patients had advanced stage cancers, only 67 (32%) presented at an early stage; 108 (53%) were receiving radiotherapy, 49 (24%) were on chemotherapy while 14 (7%) were on combined chemotherapy and radiotherapy with or without surgery; 33 (16%) patients were on analgesics and haematinics alone, whilst being worked up for radiotherapy (Table 2).
One hundred and fifty nine (76%) of the subjects reported having pain ranging from very mild to very severe. The commonest site was the abdomen (related to cancers of the colon, prostate, and cervix).
Significant physical symptoms were found in 141 patients — ulceration of the cancer site, 21 (15%); swelling at the cancer site, 17 (12%); fracture, 1 (1%); 102 (72%) lost weight (Table 2). Besides, 142 (68%) had impaired sexual functioning, out of which 86% complained of lack of sexual drive (loss of libido).
History of Mental Illness / Use of Alcohol
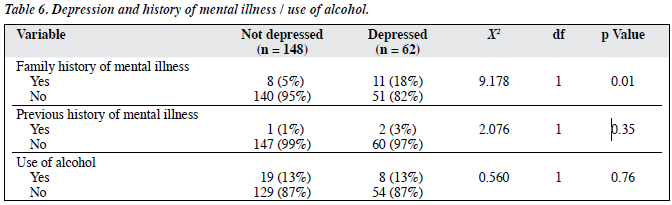
Presence of a family history of mental illness was obtained in 19 (9%) subjects, but they did not know the diagnosis. Three (1%) subjects said they had a previous history of mental illness, with descriptions suggestive of an affective illness, though none received any orthodox form of treatment. One hundred and eighty seven (89%) patients denied using alcohol, 19 (9%) said they drank on social occasions, 2 (1%) admitted they drank regularly but a little quantity at a time while 2 (1%) said they drank heavily. Their description of the quantities of alcohol was inconsistent and estimating the units consumed per week deemed unreliable. However, using the alcohol screening questionnaire, Alcohol Use Disorder Identification Test,25 none of the 23 subjects met the criteria for alcohol use disorders (Table 3).
Diagnosis of Depression
Of the 210 subjects interviewed, 62 (30%) met the DSM- IV diagnostic criteria for a major depressive episode within the past 12 months, of whom 18 were males. Sixteen had depressive symptoms but did not meet the full criteria for a depressive disorder, while 132 (63%) were not depressed. In all, 148 subjects did not meet diagnostic criteria for depression and were regarded as ‘not depressed’. There was no significant gender difference in the prevalence of depression (X2 = 5.112, p = 0.08).
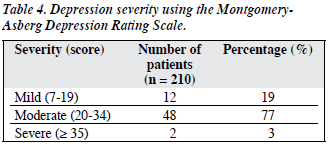
Using the MADRS scores, 12 (19%) of those depressed had mild depression, 48 (77%) were moderately depressed, while 2 (3%) had severe depression (Table 4).
Predictors of Depression
Approximately 28%, 29%, 35%, and 28% of subjects with cancer of the breast (19 / 68), cervix (17 / 59), colon / rectum (14 / 40), and prostate (12 / 43) respectively, were depressed. The prevalence of depression was not significantly related to the types of cancer (p = 0.18).
Half (50%) of the 104 subjects with advanced illness were depressed, as opposed to only 7% of the 67 with early stage cancer. There was a significantly higher prevalence of depression in those with late-stage disease (p < 0.001).
Depression occurred within the first 2 years of illness in 62% of subjects while 8% of the depressed subjects had depression after the fourth year. The duration of cancer was not significantly related to the prevalence of depression (p = 0.37).
Of 155 subjects who had pains, 55 (36%) were depressed while 7 out of 55 (13%) who had no pains were depressed, yielded a statistically significant difference (p = 0.004).
The associations between various other types of physical symptoms and the prevalence of depression were not statistically significant (p = 0.20), nor were the associations between various forms of cancer treatment (p = 0.17).
Only 19 subjects admitted having a family history of mental illness, of whom 11 (58%) indicated that the illness was depression. There was a significant association between a positive family history of mental illness and the patient having depression (p = 0.01).
Three subjects reported having had a previous episode of mental illness, of whom 2 met the diagnostic criteria for depression in this study, but the latter constituted only 3% of all the depressed subjects (p = 0.35). The use of alcohol was not significantly associated with a higher prevalence of depression (p = 0.76).
After entering the three variables significantly associated with depression (stage of cancer, presence of pains, and family history of mental illness) into the multiple logistic regression analysis, they were still significantly predictive of depression independently. Among these were the stage of cancer (p < 0.001), presence of pains (p = 0.03), and a positive family history of mental illness (p = 0.03) [Tables 5 and 6].
Depression and Quality of Life
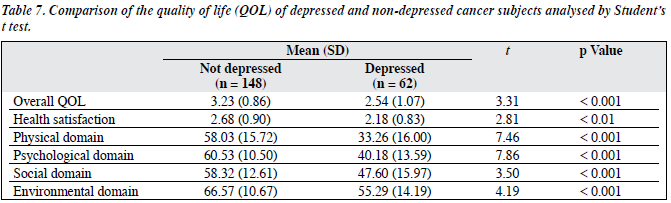
As shown in Table 7, the overall quality of life, health satisfaction, and all other quality-of-life domains of depressed cancer patients were poorer than those of their counterparts who were not depressed.
Discussion
In this study, 70% of the subjects were females, consistent with the preponderance of breast and cervical cancers in our subjects. Only 43 had cancer of the prostate (exclusively males), while cancer of the colon / rectum occurred in males and females. Breast cancer was the commonest malignancy and accounted for 68 (32%) of the patients, which was consistent with previous reports from our centre.7,26
In this study we found the prevalence of depression to be 30%. This figure was comparable to the rates reported in cancer patients from other parts of the world.8,12,27-29 Some studies however reported a much higher prevalence,7,9,30 while others indicated a lower rates.6,10 The study by Ohaeri et al7 considered depressive symptoms rather than depressive disorders and 45% of their subjects had ‘depression about their condition’. The possible explanation for the different prevalence rates between studies may be due to the instruments used, the type of subjects selected (outpatients, inpatients, terminally ill patients, elderly or adolescents), the types of cancer, and whether distinctions were made between depressive symptoms and depressive disorders.
There was no significant difference in the prevalence of depression among different types of cancer. Zabora et al31 reported that patients with lung cancer had more psychological distress than those with gynaecological cancers. However, the meaning and interpretation of having a cancer may be more important in developing psychological symptoms.32 It is possible that our subjects interpreted the diagnosis of cancer in the same way irrespective of the type or site of the disease. Zabora’s study31 also entailed a much larger sample size (4,496 subjects); absence of significant differences in our series may be related to lack of power.
Patients with an advanced stage of cancer were more depressed than those with early-stage disease, which was consistent with previous reports.8,10 It is possible that there are more physical disabilities manifest as the illness progresses and watching the effects of cancer on other patients may lead to the fear of impending death and loss of autonomy. The progression of the illness may also lead to feelings of helplessness, hopelessness, and worthlessness.
In this study, pain was strongly associated with depression, which was in keeping with studies by Massie and Holland,8 Grassi and Rosti,9 Atesci et al,33 and Breitbert.34
Patients possibly perceived the presence of pain as a feature of severity or impending death. The discomfort and loss of sleep caused by pain may also account for depression in cancer patients.
Our study did not find a statistically significant association between the duration of cancer and the prevalence of depression. However, there may be recall bias. This is in contrast to Grassi and Rosti’s9 findings, in which the prevalence of depression decreased from 47% at diagnosis to 37% 6 years later. A possible explanation for this difference may be related to the quality of care most Nigerian cancer patients receive. Rather than coming to a tertiary centre for treatment, many patients visit herbalists, traditional healers, and self-acclaimed religious healers, whose remedies have no scientifically proven efficacy.
In this study, the tendency for cancer medications to cause depression was not established. Notably, the report by Massie and Holland8 from a New York cancer centre claimed that patients on anticancer drugs which produce depression as a side-effect are at highest risk. The type of drug may therefore account for the absence of any association in our series; 36 (74%) of those receiving chemotherapy were taking a CMF (cyclophosphamide, methotrexate, 5-fluorouracil) combination and 8 (16%) were taking steroids.
The presence of physical symptoms was common, though not significantly associated with depression. It is possible that Nigerian patients do not lay much emphasis on their cosmetic appearance. It is also necessary to determine whether these physical symptoms were the direct effects of the cancers or due to co-morbid disorders.
In this study, having a previous history of mental illness was not significantly associated with depression, which is in contrast to other studies reporting an increased risk among cancer patients.8,33 A possible reason may be that only 3 (1%) of our subjects reported having had a previous psychiatric illness, 2 of whom had been depressed (constituting only 3% of all depressed subjects).
Many (87%) patients denied the use of alcohol. The few who admitted taking some could not quantify how much. Of 23 admitting to alcohol use, 19 claimed they only drank on social occasions such as naming ceremonies or weddings (common social events in Nigeria).
Significant association between a family history of mental illness and depression was found. Having a family history of mental illness is a predisposing factor, and depression was probably precipitated by cancer in subjects who were already vulnerable.
After logistic regression analysis, 3 factors (presence of pain, advanced stage of cancer, and family history of mental illness) were independent predictors of depression among cancer patients.
Our findings support previous reports that co-morbid depression in cancer patients has a negative impact on their quality of life.11-14 This was true for all the domains, pertaining to overall quality of life and the health satisfaction. Even though Jacobson et al35 cautioned that pessimism (a feature of depression) may account for patients giving negative reports about their quality of life, it is important to address this issue as it may influence the patient’s future actions (e.g. suicidal attempt).
One of the limitations of our study is that it was cross- sectional, and involved only 4 types of cancer. It is also possible that there was under-reporting of previous history of mental illness and use of alcohol, accounting for absence of associations with depression.
Our results support previous findings that depression is common among cancer patients even though under- recognised, and that pain and advanced stage disease are predictive of depression in this population. We also found that a family history of mental illness is a risk for depression in cancer patients, and that depression affects the quality of life of cancer patients negatively. Therefore government, non-governmental organisations, and corporate bodies should organise awareness / enlightenment campaigns in the form of seminars and workshops to better educate people about cancer.
Radio, television and newspaper advertisements should be monitored and regulated and false claims of treatment efficacy often emanating from non-orthodox medical practitioners should be discouraged. There should be provision of screening facilities such as mammography, recto-sigmoidoscopy and cone biopsy, so that cancers can be detected and diagnosed at an early stage when the prospect of cure is high. This will also reduce the number of patients presenting with advanced disease when risk of having depression is high.
Pain should be adequately controlled by judicious use of analgesic drugs. The fear that patients become dependent on analgesics should not deprive them of a good quality of life. Clinicians managing cancer patients should include routine psychological assessment in their evaluations, especially in those at risk of becoming depressed. This can be achieved by strengthening the consultation-liaison services in hospitals.
References
- Maguire P, Walsh S, Jeacock J, Kingston R. Physical and psychological needs of patients dying from colorectal cancer. Palliat Med 1999;13:45- 50.
- Rubin E, Rubin R, Aaronson S, editors. Neoplasia in Rubin’s pathology: Clinicopathologic foundation of medicine. 4th ed. Philadelphia: Lippincott Williams and Wilkins Company; 2004: 165-213.
- Härter M, Reuter K, Schretzmann B, Hasenburg A, Aschenbrenner A, Weis J. Co-morbid psychiatric disorders in cancer patients in acute inpatient treatment and medical rehabilitation [in German]. Rehabilitation (Stuttg) 2000;39:317-23.
- Akechi T, Nakano T, Okumura H, Ueda S, Akizuki N, Nakanishi T, et al. Psychiatric disorders in cancer patients: descriptive analysis of 1721 psychiatric referrals at two Japanese cancer center hospitals. Jpn J Clin Oncol 2001;31:188-94.
- Craig TJ, Abeloff MD. Psychiatric symptomatology among hospitalized cancer patients. Am J Psychiatry 1974;131:1323-7.
- Berard RM, Boermeester F. Psychiatric symptomatology in adolescents with cancer. Pediatr Hematol Oncol 1988;15:211-21.
- Ohaeri JU, Campbell OB, Ilesanmil AO, Ohaeri BM. Psychosocial concerns of Nigerian women with breast and cervical cancer. Psychooncology 1998;7:494-501.
- Massie MJ, Holland JC. Depression and the cancer patient. J Clin Psychiatry 1990;51(Suppl):S12-9.
- Grassi L, Rosti G. Psychosocial morbidity and adjustment to illness among long-term cancer survivors. A six-year follow-up study. Psychosomatics 1996;37:523-32.
- Kugaya A, Akechi T, Okuyama T, Nakano T, Mikami I, Okamura H, et al. Prevalence, predictive factors, and screening for psychologic distress in patients with newly diagnosed head and neck cancer. Cancer 2000;88:2817-23.
- Osoba D. Lessons learned from measuring health-related quality of life in oncology. J Clin Oncol 1994;12:608-16.
- Valente SM, Saunders JM. Diagnosis and treatment of major depression among people with cancer. Cancer Nurs 1997;20:168-77.
- Newport DJ, Nemeroff CB. Assessment and treatment of depression in the cancer patient. J Psychosom Res 1998;45:215-37.
- Jones RD. Depression and anxiety in oncology: the oncologist’s perspective. J Clin Psychiatry 2001;62(Suppl 8):S52-5,56-7.
- Williams JB, Gibbon M, First MB, Spitzer RL, Davies M, Borus J, et al. The Structured Clinical Interview for DSM-III-R (SCID). II. Multisite test-retest reliability. Arch Gen Psychiatry 1992;49:630-6.
- Shear MK, Greeno C, Kang J, Ludewig D, Frank E, Swartz HA, et al. Diagnosis of nonpsychotic patients in community clinics. Am J Psychiatry 2000;157:581-7.
- Fennig S, Craig T, Lavelle J, Kovasznay B, Bromet EJ. Best-estimate versus structured interview-based diagnosis in first-admission psychosis. Compr Psychiatry 1994;35:341-8.
- Kranzler HR, Kadden RM, Babor TF, Tennen H, Rounsaville BJ. Validity of the SCID in substance abuse patients. Addiction 1996;91:859-68.
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979;134:382-9.
- Kearns NP, Cruickshank CA, McGuigan KJ, Riley SA, Shaw SP, Snaith RP. A comparison of depression rating scales. Br J Psychiatry 1982;141:45-9.
- Thompson LW, Futterman A, Gallagher D. Assessment of late-life depression. Psychopharmacol Bull 1988;24:577-86.
- Snaith RP, Harrop FM, Newby DA, Teale C. Grade scores of the Montgomery-Asberg Depression and the Clinical Anxiety Scales. Br J Psychiatry 1986;148:599-601.
- Issa BA, Baiyewu O. Quality of life of patients with diabetes mellitus in a Nigerian teaching hospital. Hong Kong J Psychiatry 2006;16:27- 33.
- Morakinyo O, Oyelaran O. The translation factor in the cross-cultural utilization of personality questionnaires. Nigerian J Psychiatry 1982;1:5-12.
- Adewuya OA. Validation of the alcohol use disorders identification test (audit) as a screening tool for alcohol-related problems among Nigerian university students. Alcohol Alcohol 2005;40:575-7.
- Ibadan Cancer Registry. Ten commonest cancer cases in 2004. Nigeria: Department of Pathology, University of Ibadan, Ibadan.
- Hammerlid F, Ahlner-Elmqvist M, Bjordal K, Biörklund A, Evensen J, Boysen M, et al. A prospective multicentre study in Sweden and Norway of mental distress and psychiatric morbidity in head and neck cancer patients. Br J Cancer 1999;80:766-74.
- Farragher B. Psychiatric morbidity following the diagnosis and treatment of early breast cancer. Ir J Med Sci 1998;167:166-9.
- Hosaka T, Aoki T. Depression among cancer patients. Psychiatry Clin Neurosci 1996;50:309-12.
- Kissane DW, Clarke DM, Ikin J, Bloch S, Smith GC, Vitetta L, et al. Psychological morbidity and quality of life in Australian women with early-stage breast cancer: a cross-sectional survey. Med J Aust 1998;169:192-6.
- Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psychooncology 2001;10:19-28.
- Straker N. Psychodynamic psychotherapy for cancer patients. J Psychother Pract Res 1997;7:1-9.
- Atesci FC, Baltalarli B, Oguzhanoglu NK, Karadag F, Ozdel O, Karagoz N. Psychiatric morbidity among cancer patients and awareness of illness. Support Care Cancer 2004;12:161-7.
- Breitbart W. Psychiatric management of cancer pain. Cancer 1989;63(11 Suppl):S2336-42.
- Jacobson AM, de Groot M, Samson JA. The evaluation of two measures of quality of life in patients with type I and type II diabetes. Diabetes Care 1994;17:267-74.