Hong Kong J Psychiatry 2008;18:152-7
ORIGINAL ARTICLE
施用抗抑鬱藥草酸依他普侖治療馬來西亞病人的強迫症
Dr A Hatim, MPM, Department of Psychological Medicine, University of Malaya Medical Center, Kuala Lumpur, Malaysia.
Dr JS Gill, MPM, Department of Psychological Medicine, University of Malaya Medical Center, Kuala Lumpur, Malaysia.
Dr ST Jambunathan, MPM, Department of Psychological Medicine, University of Malaya Medical Center, Kuala Lumpur, Malaysia.
Dr TH Yen, MD, Psychiatric Assessment Consultation Treatment Centre, Kuala Lumpur, Malaysia.
Dr M Ariff, MD, Hospital Raja Perempuan Zainab II, Kota Bharu Kelantan, Malaysia.
Mr OM Lemming, MSc, H. Lundbeck A/S, Ottiliavej 9, DK-2500 Valby, Denmark.
Prof MZ Azhar, MPM, Department of Psychiatry, University Putra Malaysia, Selangor, Malaysia.
Address for correspondence: Dr A Hatim, Department of Psychological Medicine, University of Malaya Medical Center, Kuala Lumpur, Malaysia.
Tel: (603) 7957 0995; Fax: (603) 7957 1058; E-mail: hatim@um.edu.my
Submitted: 28 February 2008; Accepted: 5 May 2008
Abstract
Objective: This post-hoc analysis examined the efficacy and tolerability of escitalopram in the prevention of relapse in Malaysian patients with obsessive-compulsive disorder.
Participants and Methods: In Malaysia, 47 patients with obsessive-compulsive disorder were treated with open-label escitalopram (10 mg or 20 mg/day) for 16 weeks, after which the 34 responders (Yale- Brown Obsessive Compulsive Scale total decrease score, ≥ 25%) were randomised to placebo or escitalopram for 24 weeks, using a double-blind protocol.
Results: The primary efficacy analysis suggested a trend in favour of escitalopram treatment with respect to time to relapse (log-rank test, p = 0.07). A higher proportion of patients relapsed after placebo treatment (5 of 14, 36%) than with escitalopram treatment (2 of 20, 10%) [Fisher’s exact test, 2-sided; p = 0.10]. The risk of relapse was 4-fold higher for placebo than escitalopram treatment (p = 0.09). During the double-blind period, the proportion of patients reporting treatment-emergent adverse events was comparable in the 2 groups (10% in the escitalopram group vs. 14% in the placebo group); no serious events being reported.
Conclusions: This post-hoc subgroup analysis suggests that escitalopram is well tolerated in Malaysian patients with obsessive-compulsive disorder and appears to confer an advantage over placebo, in terms of time to relapse and other efficacy variables.
Key words: Citalopram; Obsessive-compulsive disorder; Recurrence; Treatment outcome
摘要
目的:本分析旨在檢視草酸依他普侖在防止馬來西亞強迫症患者復發方面的療效,及患者對藥物的耐受情況。
參與者與方法:以開放標籤方式對47位強迫症患者施用草酸依他普侖16星期(每日10 mg或 20 mg) , 繼而以雙盲法,對34位回應者(據耶魯布朗強迫症狀評量表,其症狀減輕總分 ≥ 25%)隨機施用安慰劑或草酸依他普侖24星期。
結果:主要指標分析反映,以草酸依他普侖治療較有效,該組強迫症再發與用藥期相距的時間比起安慰劑組的要長(log-rank檢驗,p = 0.07),而且安慰劑組強迫症復發的患者比例(14位中的5位,36%)較草酸依他普侖組的(20位中的2位,10%)為高(Fisher’s確切概率,雙側 p = 0.10),復發的機會比草酸依他普侖組高四倍(p = 0.09)。在雙盲法用藥期,兩組患者報告由治療引起不良事件的比例相若(用藥組10%比安慰劑組14%),亦無嚴重事故報告。
結論:本小組分析顯示,馬來西亞的強迫症患者對草酸依他普侖的耐藥情況良好,以強迫症再發的時間及其他療效可變因子來看,該藥似乎比安慰劑佔優。
關鍵詞:西酞普蘭、強迫症、復發、治療效果
Introduction
Escitalopram has been approved in many countries for the treatment of depression and anxiety disorders.1 Two recently published randomised controlled trials have demonstrated that it is also efficacious in the treatment of obsessive-compulsive disorder (OCD). One study was a 24-week, placebo-controlled fixed-dose trial,2 and showed that escitalopram 20 mg/day was associated with an earlier onset and higher response rate, a higher remission rate, improved functioning, and better tolerance. A second trial, in patients with OCD by Fineberg et al,3 involved open- label escitalopram treatment for 16 weeks, after which responders were randomised to placebo or escitalopram for 24 weeks of double-blind treatment. This study, which was conducted in 14 countries including Malaysia, showed that escitalopram was well tolerated, and that improvements in symptoms observed during the open-label period were sustained by active drug treatment during the double-blind extension period.
There is a paucity of data concerning the efficacy and adverse event (AE) profile of escitalopram treatment in Malaysian patients. Response to selective serotonin reuptake inhibitors (SSRIs) may be influenced by body weight, age, sex, and genetic makeup, and therefore can vary between individuals of different ethnic populations.4 To address this gap in knowledge, a subgroup analysis of the Malaysian patients who had participated in Fineberg et al’s3 study was performed. The results of this post-hoc subgroup analysis are also presented.
Methods
The design and conduct of this study has been described.3 The study was approved by local ethics committees and all patients gave written informed consent. In Malaysia, the study was conducted at 4 centres.
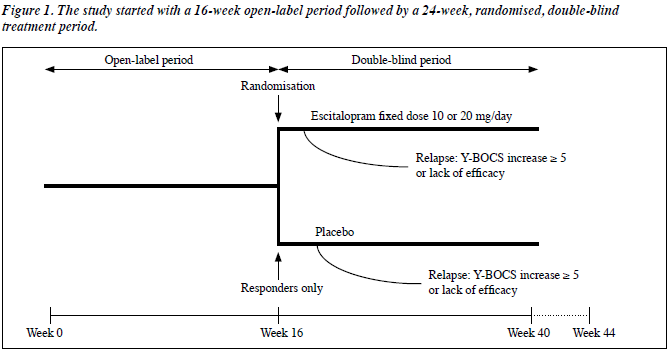
This was a relapse prevention study commencing with a 16-week open-label treatment period, followed by 24 weeks of double-blind, randomised treatment (Figure 1). During the open-label period patients received 10 mg/day escitalopram for the first week, after which the dose could be increased to 20 mg/day at a scheduled visit. Subsequently, the dose could be decreased to 10 mg/day according to the investigator’s judgement. After week 12, the dose was fixed. At the end of the 16-week open-label period, responders (those with ≥ 25% decrease from baseline in total Yale-Brown Obsessive Compulsive Scale [Y-BOCS] score) were eligible for randomisation to escitalopram (fixed dose of 10 or 20 mg/ day), or placebo in a 1:1 ratio. Non-responders (withdrawn from the study) were treated at the investigator’s discretion.
Patients randomised to placebo who were taking 20 mg/day escitalopram from week 12 in the open-label period received 10 mg/day for the first week of the double- blind period, and then placebo for the remainder of the study. Patients randomised to placebo who were taking 10 mg/day escitalopram from week 12 in the open-label period received placebo for the double-blind period. Patients randomised to escitalopram continued on the dose they had received after week 12 in the open-label period. After completion of the study, there was a 1-week period of double-blind down-tapering, during which patients on 20 mg/day escitalopram received 10 mg/day, while patients on 10 mg/day escitalopram or placebo received only placebo.
Throughout the 24-week double-blind period, the investigators evaluated relapse of symptoms; relapse was defined as either an increase in the Y-BOCS total score of ≥ 5 points from the time of randomisation, or, an unsatisfactory treatment effect as judged by the investigator. Patients who relapsed were withdrawn from the study.
Patients were enrolled from psychiatric practices, specialised clinical centres, psychiatric hospital departments, or general practices. Patients eligible for this study were outpatients between 18 and 65 years (extremes included). They had to have a primary diagnosis of OCD according to Diagnostic and Statistical Manual of Mental Disorders 4th Edition, Text Revision5 without other primary psychiatric disorders or significant somatic morbidity, and with a Y- BOCS total score ≥ 20.
Efficacy and tolerability parameters were assessed at weeks 1 and 2, and then every 2 weeks during the open-label period. In the double-blind period, efficacy and tolerability parameters were assessed 2, 4, 6, 7, and 12 weeks after randomisation, and then every 4 weeks until their last dose of double-blind treatment.
The prospectively defined primary analysis of efficacy was the time to relapse from the start date of double-blind treatment. Secondary efficacy parameters included: the proportion of relapsed patients and change in the total Y- BOCS scores. Tolerability and safety evaluations were based on spontaneously reported AEs.
Statistical Analysis
In this post-hoc subgroup analysis, the efficacy analyses were based on the full-analysis set population of Malaysian patients in the study by Fineberg et al.3 The latter consisted of all corresponding patients who took at least 1 dose of the trial medication and had at least one efficacy assessment, post-randomisation. The primary efficacy analysis comparing time-to-relapse between treatment groups (escitalopram versus placebo) was based on survival analysis methods (log-rank test and Kaplan-Meier plot). The hazard ratio was estimated on the basis of the Cox proportional hazards model. Fisher’s exact test was used to compare the proportions of relapsed patients.
Results
Patient Disposition at Baseline
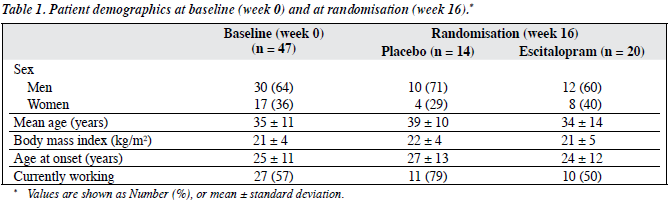
In Malaysia, 47 patients were treated (APTS I, took at least 1 dose of study medication) in the open-label period (Figure 2). Of these, 10 patients withdrew and 37 completed the open-label treatment. The reasons for withdrawal were: withdrawal of consent (7), AEs (1), protocol violation (1), and loss to follow-up (1). More men than women (30 vs. 17) participated in Malaysia, whereas in the study as a whole there were approximately equal numbers of both genders. Their mean age was 35 years and all were classified as ‘Asian’. The mean body mass index of patients from Malaysia was lower than that of the overall cohort (21 vs. 24 kg/m2), reflective of differences in ethnicity. The majority of Malaysian patients had experienced the current level of OCD symptoms for 2 to 5 years, with onset of symptoms ranging from 1 to 40 years old. At baseline, 28 (57%) patients were currently employed, and 2 (4%) had taken sick leave within the previous 3 months. Their mean Y-BOCS total score at baseline was 26.3, representing moderate-to-severe OCD, which was in line with a baseline total Y-BOCS score of 26.4 for the whole study population.
Patient Disposition at Randomisation
Thirty four of the 37 patients who completed the open- label period were randomised to double-blind treatment, 20 to escitalopram and 14 to placebo. Of the 20 patients randomised to escitalopram, 18 completed the course and 2 withdrew (both due to lack of efficacy). Of the 14 patients randomised to placebo, 9 completed the course and 5 withdrew (all due to lack of efficacy).
In the escitalopram group, 12 patients were men, and 8 were women (Table 1), as opposed to 10 men and 4 women receiving placebo. The mean age of patients randomised to escitalopram was 34 years, and 39 years for those randomised to placebo. At randomisation, 50% and 79% of the patients randomised to escitalopram and placebo respectively were currently employed. In the double-blind period, 17 of the 20 patients in the escitalopram group received 20 mg/day.
Efficacy
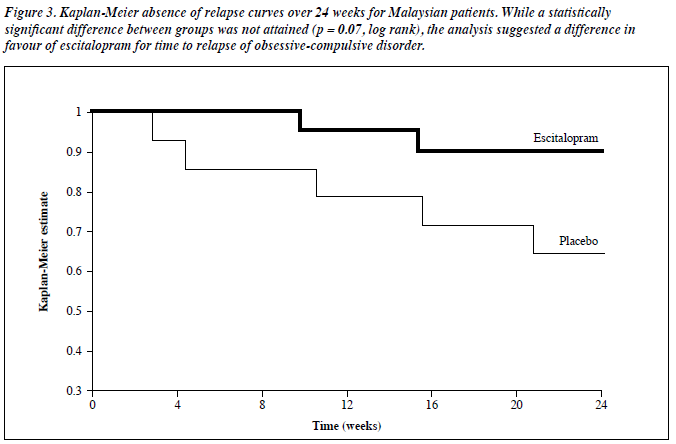
The primary efficacy analysis suggested a treatment difference in favour of escitalopram for the time to relapse of OCD (Figure 3, log-rank test, p = 0.07). A higher proportion of patients taking placebo treatment relapsed (5/14; 36% versus 2/20, 10%) [Fisher’s exact test, 2-sided; p = 0.10]. The Cox proportional hazards model gave an estimated hazard ratio of 4.06 (p = 0.09). Thus, the risk of relapse for those treated with placebo appeared to be 4 times that of those treated with escitalopram.
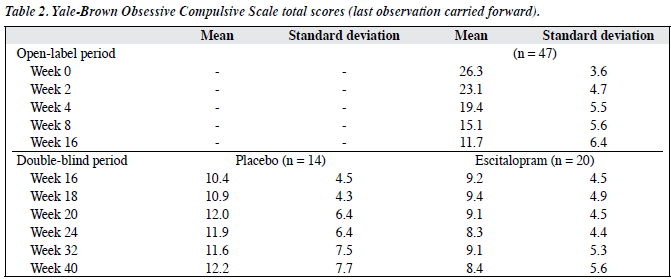
During the open-label period, the mean Y-BOCS total score for the Malaysian patients decreased from 26.3 at baseline to 11.7 at week 16, which amounts to a clinically relevant decrease of 14.6 points (Table 2). During the double-blind period, the mean change in total Y-BOCS score from the start of double-blind treatment (week 16) to week 40 entailed a worsening of 1.9 in the placebo group and an improvement of 0.9 in those taking escitalopram, but this difference was not statistically significant.
The proportion of remitters (defined as a total Y-BOCS score ≤ 10) during the double-blind period decreased in the placebo group (from 64% at week 16 to 57% at week 40). In the escitalopram group, the percentage increased (from 65% at week 16 to 70% at week 40).
Safety
In the open-label period, 22 (47%) of the patients reported 36 AEs; the majority being mild to moderate, and none were serious. One patient withdrew due to mild throat tightness. The AEs with the highest incidence were lethargy and nausea (both reported by 5 patients).
In the double-blind period, 2 (14%) patients in the placebo group reported dizziness, which was termed a treatment-emergent adverse event (TEAE). Two (10%) patients in the escitalopram group reported 5 TEAEs (delayed ejaculation, dizziness, fall, hypertension, laceration), all of which were considered to be mild. There were no serious AEs, and no patients withdrew due to AEs.
Discussion
To our knowledge, this is the first publication to investigate the use of an escitalopram in Malaysian patients with OCD. It is recognised that the responses to drugs often show wide inter-individual variations. One of the major causes being genetic polymorphism of drug-metabolising enzymes in the liver.6 The enzymes primarily involved in escitalopram metabolism are the cytochrome P450 isozymes: CYP2C19, CYP2D6, and CYP3A4.7 The incidence of CYP2C19 poor metaboliser phenotype is markedly higher among Asian populations (13-23%) than in Caucasians (2-5%),8 and Yin et al9 have previously postulated a correlation between CYP2C19 polymorphism and the clinical AEs of citalopram (of which escitalopram is its S-enantiomer and main pharmacologically active agent).
The results of this subgroup analysis suggest that escitalopram is well tolerated in Malaysian patients with OCD. No serious AEs were reported throughout the study. Though one patient withdrew due to an adverse event (throat tightness), it was classified as mild. The proportion of patients experiencing TEAEs was comparable in the escitalopram and placebo groups, both during the double- blind period (10% vs. 14%), and throughout the study. No safety issues were reported among Malaysian patients outside the expected and known side-effects of escitalopram. These findings are of interest, given the evidence of a higher incidence of CYP2C19 poor metaboliser phenotypes among Asians. However, our sample size may have been too small to yield broad generalisations about the safety profile of escitalopram among Malaysian patients, vis-a-vis other ethnic populations.
Consistent with the results of the main analysis,3 this subgroup showed an advantage for escitalopram treatment over placebo in terms of time to relapse as well as other efficacy variables. Given the relatively small sample size relative to the main study, the primary analysis of this subset showed significance at the 10% level only (p = 0.07, log- rank test). This was hardly surprising, considering that a subgroup analysis did not have the same statistical power as the main analysis.
For several reasons, caution should be exercised in interpreting these results. First, all Malaysians were categorised as “Asian” in ethnicity; however, Malaysia is a multi-ethnic country, comprising of Malays, Chinese, and Indians. Second, serum drug level measurements were not carried out, and therefore, the correlation between clinical response and ethnicity cannot be confirmed. In conclusion, this post-hoc subgroup analysis suggests that escitalopram is well tolerated in Malaysian patients with OCD and appears to confer an advantage over placebo in terms of time to relapse and other efficacy variables. Larger studies are needed to substantiate these findings.
Acknowledgements
The authors gratefully acknowledge the contribution of the each of the following study centres: University of Malaya Medical Center (Prof Dr Mohd Hussain Habil, Assoc Prof Nor Zuraida Zainal, Dr Francis Low Chee Chan, and Mdm Tang Suet Mooi), University Putra Malaysia (Dr Khin Ohnmar Naing and Ms Haslinda Hairi), Hospital Raja Perempuan Zainab II (Dr Suarn Singh, Dr Raziffah Abdul Rahman, Dr Khairi Chemat, Nurse Yew, Ms Hjh Eshah BT Ahmad), Psychiatric Assessment Consultation Treatment Centre (Dr Au Yong Koon Choon and Ms Angerie How). The authors received no additional funding for this report, and wish to thank Lundbeck Export A/S for their assistance in manuscript preparation. Ole Michael Lemming is a full- time employee of H. Lundbeck A/S. This study was funded by H. Lundbeck A/S.
References
- Owens MJ, Knight DL, Nemeroff CB. Second-generation SSRIs: human monoamine transporter binding profile of escitalopram and R- fluoxetine. Biol Psychiatry 2001;50:345-50.
- Stein DJ, Andersen EW, Tonnoir B, Fineberg N. Escitalopram in obsessive-compulsive disorder: a randomized, placebo-controlled, paroxetine-referenced, fixed-dose, 24-week study. Curr Med Res Opin 2007;23:701-11.
- Fineberg NA, Tonnoir B, Lemming O, Stein DJ. Escitalopram prevents relapse of obsessive-compulsive disorder. Eur Neuropsychopharmacol 2007;17:430-9.
- Mao PX, Tang YL, Jiang F, Shu L, Gu X, Li M, et al. Escitalopram in major depressive disorder: a multicenter, randomized, double-blind, fixed-dose, parallel trial in a Chinese population. Depress Anxiety 2008;25:46-54.
- Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994.
- Yu BN, Chen GL, He N, Ouyang DS, Chen XP, Liu ZQ, et al. Pharmacokinetics of citalopram in relation to genetic polymorphism of CYP2C19. Drug Metab Dispos 2003;31:1255-9.
- Rao N. The clinical pharmacokinetics of escitalopram. Clin Pharmacokinet 2007;46:281-90.
- de Morais SM, Wilkinson GR, Blaisdell J, Nakamura K, Meyer UA, Goldstein JA. The major genetic defect responsible for the polymorphism of S-mephenytoin metabolism in humans. J Biol Chem 1994;269:15419-22.
- Yin OQ, Wing YK, Cheung Y, Wang ZJ, Lam SL, Chiu HF, et al. Phenotype-genotype relationship and clinical effects of citalopram in Chinese patients. J Clin Psychopharmacol 2006;26:367-72.