Hong Kong J Psychiatry 2004;14(2):2-7
ORIGINAL ARTICLE
Dr Ki-Yan Mak, MBBS, FRCPsych, DPM, MHA, FHKAM, FHKCPsych, Private Practice, Hong Kong, China.
Dr Samuel Tze-Kin Lai, MBBS, MRCPsych, FHKAM, FHKCPsych, Private Practice, Hong Kong, China.
Address for correspondence: Dr Ki-Yan Mak, Private Practice, Room 704-5, Alliance Building, 130-136 Connaught Road Central, Hong Kong, China.
Submitted: 1 August 2003; Accepted: 8 June 2004
Abstract
Objective: To evaluate the clinical use of amisulpride in Hong Kong.
Patients and Methods: In this descriptive retrospective uncontrolled open-label intent-to-treat study, the clinical records of the first 100 Chinese patients treated with amisulpride in a psychiatric outpatient clinic were analysed. The following data were extracted: socio-demographic information, diagnosis and duration of the psychiatric disorder being treated, psychotropic medications used, and response to treatment.
Results: More than half of the study patients had schizophrenia or delusional disorder. The overall efficacy of amisulpride was 54%. Relatively low dosages were used, with only 15% of patients taking 200 mg or more per day. Although women appeared to respond better than men, reports of hormonal side effects such as menstrual irregularities and galactorrhoea were noted. The incidence of extrapyramidal symptoms was low, and there were almost no anticholinergic side effects.
Conclusions: Amisulpride appears to be an effective antipsychotic medication for Chinese patients treated in an outpatient clinic, with few side effects.
Key words: Amisulpride, Psychosis, Schizophrenia
Introduction
For evaluation of the efficacy of a novel medication, the gold standard of testing is the randomised controlled trial (RCT), as recommended by the Cochrane Collaboration in the UK. However, there are difficulties in applying this stan-dard to clinical practice.1 This is because the medication dosage and duration of treatment are often outside the specified experimental range, and many patients do not fulfil the inclusion criteria of the RCT standardised protocol.
For evaluation of the efficacy of a novel medication, the gold standard of testing is the randomised controlled trial (RCT), as recommended by the Cochrane Collaboration in the UK. However, there are difficulties in applying this stan-dard to clinical practice.1 This is because the medication dosage and duration of treatment are often outside the specified experimental range, and many patients do not fulfil the inclusion criteria of the RCT standardised protocol.
Furthermore, patients involved in RCTs are often aware that they are being assessed for a new medication and may be more compliant with the prescribed regimens than is usual in clinical practice. Therefore, there is now a trend to com-plement RCTs with naturalistic uncontrolled studies in the form of an audit of the clinical use of novel medications in hospitals and outpatient clinics.
Amisulpride is a relatively novel antipsychotic agent, and its efficacy among patients with schizophrenia has been established by RCTs.2-4 The propensity of amisulpride to cause extrapyramidal side effects has been established.5 Amisulpride is thought to cause antagonism of dopamine D2 and D3 receptors6 and is useful for negative symptoms.7 Amisulpride has been available in Hong Kong since July 2000. Therefore, it is appropriate to review its clinical use for psychiatric patients in the local setting.
Patients and Methods
This was a descriptive retrospective uncontrolled open-label intent-to-treat study. The setting was a psychiatric outpatient clinic, with 3 general psychiatrists treating all types of psychiatric disorders. The records of the first 100 Chinese patients using amisulpride were analysed. The analysis was performed from April to September 2002. The following data were extracted: socio-demographic data, clinical diagnosis and duration of the disorder being treated, types of psychotropic medications used, and response to treatment.
Results
There were 39 men and 61 women included in the study. Their ages ranged from 20.0 to 87.0 years (mean, 38.0 ± 13.5 years); 6 patients were older than 65 years.
The majority of patients had a psychotic disorder such as schizophrenia or delusional disorder (Table 1). Diagnoses were made according to the International Classification of Diseases (ICD) version 9 or 10 depending on the year of presentation of the patient.
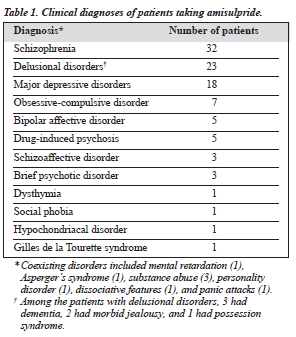
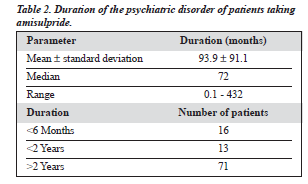
Many of the patients with major depressive disorders had paranoid features but did not fulfil the full criteria for schizophrenia or delusional disorder. Most of the patients with obsessive compulsive disorder had bizarre thoughts or behaviour. Table 2 shows the duration of the disorder from the first episode to the time of starting amisulpride.
Seventy eight patients had florid symptoms and 22 had residual symptoms. ‘Florid symptoms’ was a global subjective description, and was not restricted to psychotic disorders. For psychoses, florid symptoms referred to positive symptoms and bizarre behaviour.
For other disorders, symptom floridity referred to active symptoms of that disorder. Among the 66 patients with a psychotic disorder, 17 had an illness duration of less than 2 years, while 49 had had the disorder for more than 2 years. The number of coexisting morbidities was low — 4 patients had diabetes mellitus, 3 had thyroid disease, 2 had hypertension, 2 had coronary heart disease, 2 had chronic lung disease, 1 had uterine fibroid, 1 had carcinoma, 1 had gout, 1 had sleep apnoea, and 1 had macu-lar degeneration.
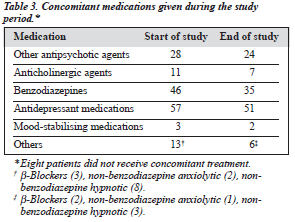
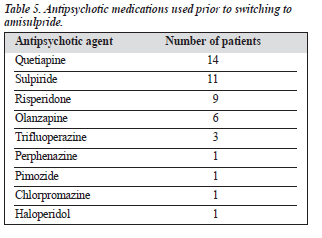
Table 3 shows the additional pharmacological treatment received by the patients at the beginning and end of the study, Table 4 shows the concomitant antipsychotic agents used during the study, and Table 5 shows the antipsychotic agents used prior to switching to amisulpride.
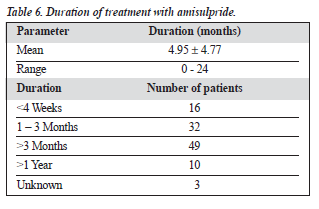
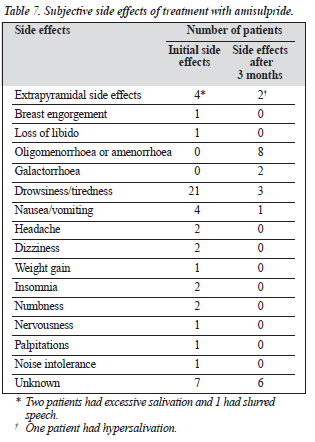
The mean duration of amisulpride treatment was 4.95 ± 4.77 months (Table 6). Among the subjective side effects were menstrual irregularities and galactorrhoea (Table 7). The incidence of extrapyramidal side effects was low, and there were almost no anticholinergic side effects.
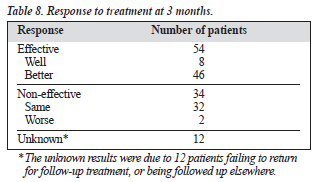
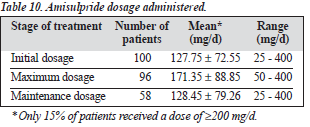
At 3 months, the drug was effective in 54% of patients (Table 8). Table 9 shows the study outcomes. Table 10 summarises the amisulpride dosage used during the study; 15% of patients received amisulpride at a dosage of at least 200 mg/d.
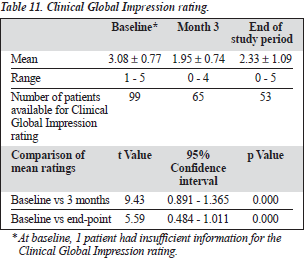
Table 11 shows the Clinical Global Impression ratings. In a comparison between patients who responded to amisulpride and those who did not (Table 12), treatment response was more likely in women than men.
DISCUSSION
The overall efficacy of amisulpride in this study was 54%, but this rate could be slightly lower if the 10% of patients with an uncertain prognosis were taken into account. However, this group of patients would probably not have had an adequate trial of the medication, which is inevitable in this type of study. This result for amisulpride should be considered therapeutic, taking into account the type of study with a mix of atypical diagnoses, different chronicities, and refractoriness to other antipsychotic medications, as is found in clinical practice. The efficacy of amisulpride was higher in this study than the finding of 44% for patients with non-refractory schizophrenia in a study from the UK.8
The main treatment categories were schizophrenia and delusional disorder and these 2 diagnoses appeared to be the most responsive to amisulpride. Other responsive psy-chotic conditions included schizoaffective disorders, drug-induced psychosis, and brief psychotic episode. On the other hand, the non-psychotic conditions such as obsessive-compulsive disorder (usually patients with strange beliefs and behaviour) and some anxiety disorders were not as responsive to this medication. Bipolar affective disorder (in its hypomanic state) was not responsive to the medication, possibly due to the relatively low dosage used (only 15% of the patients were taking more than 200 mg, and the maxi-mum dose was 400 mg). However, taking the 9 diagnostic categories into consideration, the overall analysis did not show any statistical significance, and the absolute number for most categories (except for schizophrenia and delusional disorder) was too small for individual analysis.
The above findings are relatively consistent with those of Lecrubier et al who found that amisulpride was effective for schizophrenia and acute psychosis.9 On the other hand, Amore and Jon found that amisulpride in low doses had a faster response in patients with dysthymia or double depres-sion compared with sertraline,10 although this therapeutic effect was not notable in the present study.
It should be noted that the mean daily dose used initially and for maintenance was approximately 150 to 200 mg. It may be that as the setting was an outpatient clinic, the clinical drug regimen did not correspond to that of a hospital setting.
Amisulpride appeared to be an effective antipsychotic medi-cation for Chinese patients treated in an outpatient clinic, including those who were not responsive to other atypical antipsychotic agents. The drug was effective in combating drug-induced psychotic conditions, and a trial may be worth-while for non-responsive bipolar affective and anxiety disorders. The side effects were relatively few, with virtu-ally no anticholinergic side effects. Extrapyramidal side ef-fects were rare, but hormonal changes did occur among the female patients.
Switching to amisulpride from other antipsychotic drugs was flexible, although there were no detailed analyses of the reason for or method of switching in this study. Clinical experience suggests that directly switching without taper-ing is effective, especially when the original antipsychotic dosage was not too high.
Since amisulpride does not have anticholinergic effects, anticholinergic medications were prescribed for the initial period to avoid rebound side effects of the previous anti-psychotic agents. There were no complaints of such side effects as dry mouth or constipation. The main side effects appeared to be hormonal — for example, oligomenorrhoea or amenorrhoea and galactorrhoea. On the other hand, the drowsiness or sleepiness that were common at the start of the study (possibly due in part to the previous anti-psychotic medications) were greatly decreased after switching to amisulpride. As expected, the incidence of extrapyramidal side effects was low, in agreement with the study by Perrault et al,5 but this was also true for other atypical antipsychotic agents. There was no evidence of marked weight gain, and the medication seemed to be safe when given to patients with diabetes mellitus.There were no major adverse events recorded except for the death of a woman with serious marital problems who committed suicide by overdose of a large amount of various psycho-tropic medications. Interestingly, most of the patients (more than 80%) switched from other atypical neuroleptics, suggesting that there could still be improvements for many of these patients. Furthermore, the mean dose used in this study was relatively low compared with other studies. In addition to the fact that these patients were outpatients rather than inpatients, it may be that this medication is particularly beneficial for patients with schizophrenia with negative symptoms. Unfortunately, the present study did not make a detailed analysis of this indication, nor was there any detailed analysis of the effects of different doses.
Finally, nearly half of the patients had taken the medi-cation for more than 3 months, and 10% for more than 1 year. This suggests that tolerance to the medication is good, but further exploration is indicated. This is consistent with the conclusions of a meta-analysis by Leucht et al that amisulpride is associated with lower non-compliance due to adverse events than conventional antipsychotics.3
Study Limitation
Many of the limitations of this study have been mentioned, but further studies with a larger patient population could provide more detailed results. Study of more patients who fulfil the criteria for treatment-resistant schizophrenia would help in the analysis of the effects of amisulpride in this refractory group.
In the present study, the change of dose of the concomi-tant medications were not analysed. As such variables could also affect the response of the patients to amisulpride, it would be useful to include these confounding factors in future studies. Thirty seven patients in this study defaulted or refused treatment, and the data of some of these patients could provide valuable information on treatment outcome.
The Clinical Global Impression is a crude assessment tool, and the judgement according to the clinical records was subjective. However, these are inherent deficits of natu-ralistic clinical studies. Comparison between responders and non-responders could partly neutralise these problems. Furthermore, the quality of life and functional disabilities (including cognitive functions) of the patients before and after taking the medications were not studied, and these items need further attention.11,12 In the future, the health econom-ics aspect of the medication could be studied, and com-parison between different clinical settings could also shed light on the optimal use of such an atypical medication in the treatment of psychotic disorders.
Conclusion
Amisulpride appeared to be an effective antipsychotic medi-cation for Chinese patients treated in an outpatient clinic, including those who were not responsive to other atypical antipsychotic agents. The drug was effective in combating drug-induced psychotic conditions, and a trial may be worth-while for non-responsive bipolar affective and anxiety disorders. The side effects were relatively few, with virtu-ally no anticholinergic side effects. Extrapyramidal side ef-fects were rare, but hormonal changes did occur among the female patients.
References
1. Gilbody S, Wahlbeck K, Adams C. Randomized controlled trials in schizophrenia: a critical perspective on the literature. Acta Psychiatr Scand 2002;105:243-251.
2. Moller HJ, Boyer P, Fleurot O, Rein W. Improvement of acute exacer-bations of schizophrenia with amisulpride: a comparison with haloperidol. PROD-ASLP Study Group. Psychopharmacology (Berl) 1997;132:396-401.
3. Leucht S, Petschel-Walz G, Engel RR, Kissling W. Amisulpride, an unusal ‘atypical’ antipsychotic: a meta-analysis of randomized con-trolled trials. Am J Psychiatry 2002;159:180-190.
4. Leucht S. Amisulpride — a selective dopamine antagonist and atypical antipsychotic: results of a meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol 2004;7 (Suppl 1):15-20.
5. Perrault GH, Depoortere R, Morel E, Sanger D, Scarron B. Psycho-pharmacological profile of amisulpride: an antipsychotic drug with
presynaptic D2/D3 dopamine receptor antagonist activity and limbic selectivity. J Pharmacol Exp Ther 1997;280:73-82.
6. Schoemaker H, Claustre Y, Fage D, et al. Neurochemical characteris-tics of amisulpride, an atypical dopamine D2/D3 receptor antagonist with both presynatic and limbic selectivity. J Pharmacol Exp Ther 1997; 280:83-97.
7. Boyer P, Lecrubier Y, Puech AJ, Dewailly J, Aubin F. Treatment of negative symptoms in schizophrenia with amisulpride. Br J Psychiatry 1995;166:68-72.
8. Taylor D, Mir S, Mace S. Olanzapine in practice: a prospective natu-ralistic study. Psychiatr Bull 1999;23:178-180.
9. Lecrubier Y, Azorin M, Bottai T, et al. Consensus on the practical use of amisulpride, an atypical antipsychotic, in the treatment of
schizophrenia. Neuropsychobiology 2001;44:41-46.
10. Amore M, Jon MC. Faster response on amisulpride 50 mg versus sertraline 50-100 mg in patients with dysthymia or double depression: a randomized, double-blind, parallel group study. Int Clin Psychopharmacol 2001;16:317-324.
11. Colonna L, Saleem P, Dondey-Nouvel L, Rein W. Long-term safety and efficacy of amisulpride in subchronic or chronic schizophrenia. Amisulpride Study Group. Int Clin Psychopharmacol 2000;15:13-22.
12. Carriere P, Bonhomme D, Lemperiere T. Amisulpride has a superior benefit/risk profile to haloperidol in schizophrenia: results of a multicentre, double blind study (the Amisulpride Study Group). Eur Psychiatry 2000;15:3221-329.