Hong Kong J Psychiatry 2005;15(3):82-88
ORIGINAL ARTICLE
Dr Samir Kumar Praharaj, MBBS, DPM, Resident, Central Institute of Psychiatry, Kanke, Ranchi, 834 006, India.
Dr Daya Ram, MBBS, MD, Professor of Psychiatry, Central Institute of Psychiatry, Kanke, Ranchi, 834 006, India.
Dr Manu Arora, MBBS, MD, DPM, Senior Resident, Central Institute of Psychiatry, Kanke, Ranchi, 834 006, India.
Address for correspondence: Dr Samir Kumar Praharaj, Central Institute of Psychiatry, Kanke, Ranchi, 834 006, India.
Tel: (91 651) 223 1689;
E-mail: samirpsyche@yahoo.co.in
Submitted: 5 April 2005; Accepted: 15 February 2006
Abstract
Objective: To assess the effect of medication on neurological abnormalities in patients with bipolar affective disorder.
Patients and Methods: Neurological abnormalities were examined in 30 drug-free patients and 30 drug-treated patients meeting Diagnostic and Statistical Manual of Mental Disorders, 4th edition diagnostic criteria for bipolar affective disorder, and 20 age- and sex-matched controls, using the Extended Standard Neurological Assessment Instrument. Mania and depression were assessed using the Young Mania Rating Scale and the Hamilton Rating Scale for Depression, respectively. Side effects of medications were assessed using the Udvalg for Kliniske Undersøgelser Side Effect Rating Scale.
Results: Patients with bipolar affective disorder had higher mean total scores on the Extended
Standard Neurological Assessment Instrument (drug-free group 22.83 ± 11.04, drug-treated group 22.00 ± 10.23) than controls, who did not score on any items of the neurological battery.
There was a significant excess of hard signs and involuntary movements in the drug-treated group compared with the drug-free group, and a significant positive correlation between hard signs and the involuntary movements score and the neurological subscore on Udvalg for Kliniske Undersøgelser Side Effect Rating Scale in the drug-treated group.
Conclusion: The presence of neurological signs in drug-free patients with bipolar affective disorder suggests that neurological abnormalities may occur independently of medication effects. Side effects of drug treatment for bipolar affective disorder may contribute to the prevalence of neurological abnormalities in this patient population.
Key words: Bipolar disorder, Dyskinesia, drug-induced, Neurologic manifestations
Introduction
There has been a steady increase in interest and appreciation of the biological underpinnings of mood disorders. Bipolar disorder appears to be related to anatomic abnormalities in the medial temporal lobe, in particular the amygdala, pre- frontal cortex and cerebellum.1 Recent magnetic resonance imaging findings support a neurodevelopmental etiology for bipolar affective disorder, at least in a subgroup of patients, as for schizophrenia. The frequency of medial temporal lobe (hippocampus/amygdala complex) hypoplasia noted in pa- tients with bipolar affective disorder was similar to that seen in schizophrenia, and correlated with the degree of cogni- tive impairment.2
A meta-analysis by Videbech3 showed an increased ventricle/brain ratio and other signs of cerebral atrophy, as well as an increased frequency of signal hyperintensity, in the frontal lobes and basal ganglia of patients with bipolar affective disorder. Shioiri et al4 reported a higher incidence of cavum septum pellucidum in subjects with bipolar affec- tive disorder than controls, but lower than in subjects with schizophrenia.
Neurological soft signs (NSS) have been reported in mini- mal brain dysfunction,5 emotionally unstable character,6 heavy polydrug users,7 borderline personality disorder,8 obsessive compulsive disorder,9 and consistently in individ- uals with schizophrenia.10,11 Nasrallah et al12 found that NSS are as common in individuals with mania as in those with schizophrenia. An excess of neurological signs in adults with bipolar affective disorder has also been reported by other researchers.13-15 Basu et al16 reported similar findings in ado- lescents with mania. These soft signs are thought to reflect diffuse brain dysfunction. However, it has been suggested that although diffuse, NSS may reflect selective dysfunc- tion in areas of motor coordination, integrative sensory function, and complex motor task coordination.17
One area of interest has been the role of medication in the expression of neurological abnormalities. Although few studies have been able to exclude the influence of medica- tion in the appearance of neurological abnormalities,6,18 a comprehensive review by Heinrichs and Buchanan17 con- cluded that medications do not seem to alter neurological signs in most cases of schizophrenia. Dazzan and Murray19 completed a meta-analysis and reported an excess of NSS in patients with first-episode psychosis, particularly in the areas of motor coordination and sequencing, sensory inte- gration and developmental reflexes. This was thought to be associated with a specific laterality pattern. Wong et al20 re- ported that the use of neuroleptics increased soft signs in patients, while Mukherjee et al13 found a strong correlation between the duration of cumulative neuroleptic exposure and the presence of neurological abnormalities in patients with bipolar affective disorder. A further study21 showed that patients with bipolar disorder receiving treatment with an- tipsychotic agents performed poorly on cognitive measures compared with a control group. However, most studies failed to control for medication status in the study design.
This study was designed to gather further information on the clinical, sociodemographic, psychopathological correlates and the effect of medications on neurological abnormalities in bipolar affective disorder.
Patients and Methods
The study was a cross-sectional, hospital-based study, conducted at the Central Institute of Psychiatry, Ranchi, India. Sixty patients with bipolar affective disorder (30 drug- free, 30 drug-treated) and 20 age- and sex-matched controls were recruited for the study. Inclusion criteria for the patient groups were: inpatients or outpatients of either sex; aged between 18-55 years; meeting Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) diag- nostic criteria for bipolar affective disorder; with a current episode of mania or depression; able to cooperate with testing. Drug-free was defined as not having been taking any psychotropic medications in the past month. Drug- treated was defined as on psychotropic medication for at least one month. Patients with organic brain syndrome, any other major psychiatric illness, mental retardation, or who had received electroconvulsive therapy within the previous 6 months were excluded. Control subjects were workers at the Central Institute of Psychiatry. Exclusion criteria for the control group included a history of psychosis, affective disorder, significant head injury, any major neurological disorder, epilepsy or major physical illness. A history of sub- stance abuse or dependence, or a family history of major mental disorder were further exclusion criteria for this group.
The severity of illness was assessed using the 11-item, clinician-administered Young Mania Rating Scale (YMRS)22 and the 24-item clinician-administered Hamilton De- pression Rating Scale (HDRS),23 both of which have good psychometric properties. A general health questionnaire (GHQ-5)24 was used to screen for psychiatric morbidity in the control group. The 5-item GHQ-5 (containing items 14, 38, 42, 49 and 54 of the original GHQ and designed by the original authors) has been reported to have a sensitivity of 86% and specificity of 89%.24
The Extended Standard Neurological Assessment Instrument (ESNAI)25 was used to assess neurological abnormalities. The ESNAI is a comprehensive battery for this purpose, consisting of 44 items over four domains (motor 26 items, cognitive function 6 items, reflexes 7 items, sensory 5 items), and includes both hard and soft neuro- logical signs. In a previous study, inter-rater reliability for the neurological assessment between the examiner and two other physicians was (intra-class correlation) 0.87 and 0.97, respectively.25 A few minor modifications were made to make items appropriate for Indian patients (eg, for item 20, tying a rope for males or tying hair for females instead of tying a shoelace as most patients did not wear shoes). The Udvalg for Kliniske Undersøgelser (UKU) Side Effect Rating Scale26 was used to assess side effects due to psychotropic medications.
Data Analysis
Data were analysed using the statistical software package Statistical Package for the Social Sciences 10.1. Control subjects were excluded from analysis as none had scored on any items of the scales used. Differences within the patient group in sociodemographic variables, and neuro- logical scores between drug-free and drug-treated subgroups were analysed using the Chi-squared test and the in- dependent samples t test, respectively. Pearson correlation coefficients were calculated between clinical variables, the YMRS score and UKU scores, and neurological scores. Analysis of variance with post hoc Bonferonni testing was used to assess differences between the various drug-treated groups on the neurological scores. Discriminant analysis was completed to examine whether neurological scores were able to classify the different patient subgroups.
Results
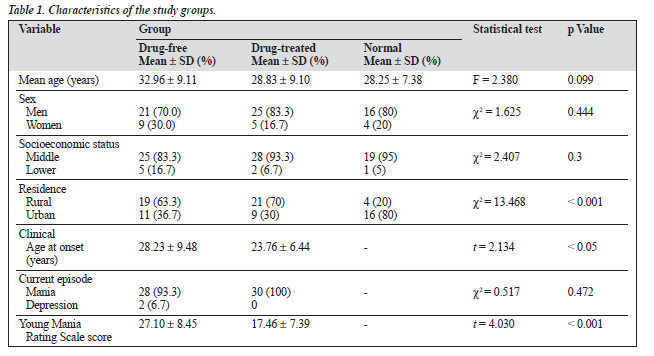
Characteristics of the study participants and differences between the groups are shown in Table 1. The mean ± SD age of the patients was 32.96 ± 9.11 years in the drug-free group and 28.83 ± 9.10 years in the drug-treated group, which was similar to controls (28.25 ± 7.38 years). There was a high representation of males (76.7%) overall but the com- position of the groups was similar. The mean age of onset of illness overall was 26 ± 8.64 years (range 13-50), being comparable in the drug-free (28.33 ± 9.48 years) and the drug-treated groups (23.76 ± 6.44). The mean number of bipolar episodes was 2.62 ± 2.04 (range 1-10). There was a significant difference (p < 0.001) in the mean YMRS scores between the drug-free and drug-treated groups.
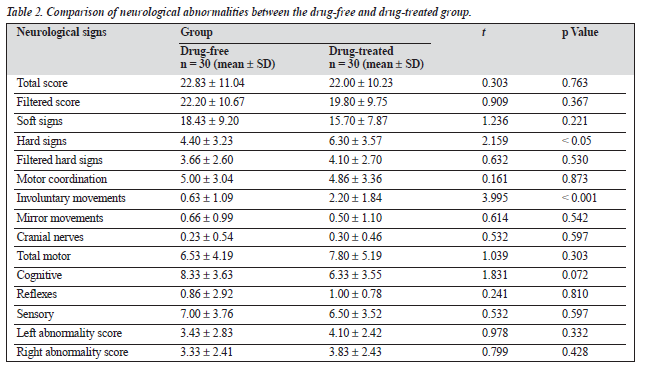
The mean total score on the neurological assessment battery was similar for both patient groups, with a mean of 22.83 ± 11.04 for the drug-free group and 22.00 ± 10.23 for the drug-treated group. There was a significant excess of hard signs (p < 0.05) and involuntary movement scores (p < 0.001) in the drug-treated group compared with the drug-free group (Table 2). In terms of educational status, patients who had less than 10 years of formal education had significantly higher mean scores (p < 0.01) in total score (t = 4.041), filtered score (t = 4.195), soft signs (t = 4.203), filtered hard signs (t = 3.222), motor coordination (t = 3.547), total motor (t = 2.930), cognitive score (t = 4.593) sensory (t = 3.537), left abnormality score (t = 3.139) and right abnormality score (t = 2.686), and significantly higher mean scores (p < 0.05) in hard signs (t = 2.506), than those with 10 or more years of formal education.
Significantly higher mean scores (p < 0.01) were also seen in residents of rural areas than those of urban areas in the total score (t = 4.249), filtered score (t = 4.060), soft signs (t = 4.034), hard signs (t = 3.550), filtered hard signs (2.971), motor coordination scores (t = 4.111), involuntary movements score (t = 2.832), cranial nerve score (t = 3.432), total motor score (t = 4.391), cognitive (t = 0.119), sensory (t = 4.365), left abnormality score (t = 3.186) and right ab- normality score (t = 3.910).
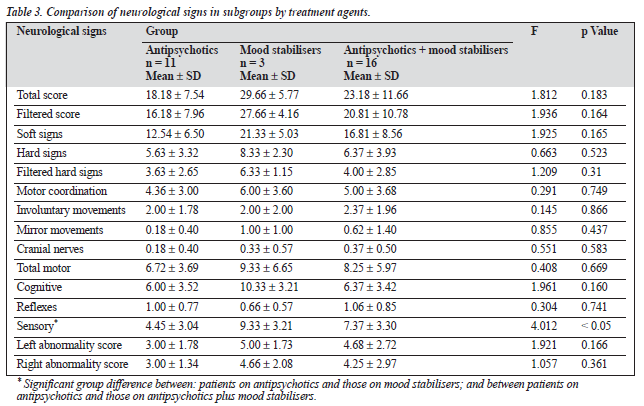
Of the drug-treated group (n = 30), 36.6% had been prescribed an antipsychotic agent only, 10% had been prescribed a mood stabiliser only, and the remainder (53.4%) had been prescribed both an antipsychotic agent and a mood stabiliser. No significant differences were seen between these three groups on the neurological signs scores, with the ex- ception of the sensory domain scores (Table 3). Significantly higher scores were found in the groups receiving mood stabilisers only, and mood stabilisers plus antipsychotic, com- pared to the group receiving antipsychotics only (F = 4.012, p < 0.05).
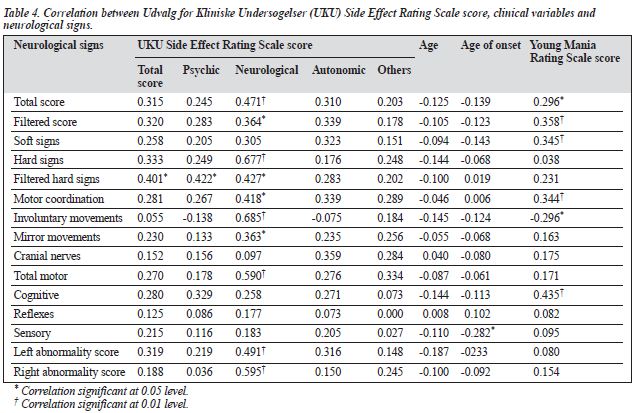
The neurological subscore of the UKU scale correlated positively with total score, hard signs, involuntary move- ments, total motor score, left and right abnormality score (p < 0.01) and with filtered score, filtered hard signs, motor coordination, and mirror movements (p < 0.05) [Table 4]. The autonomic and other subscores of the UKU scale showed no correlation with any of the neurological scores. Current age and number of episodes did not correlate significantly with any of the neurological signs. Age of onset of illness correlated negatively with the sensory domain of the neuro- logical battery. The YMRS scores correlated positively with total score (p < 0.05) and with filtered score, soft signs, motor coordination and cognitive scores (p < 0.01), and were negatively correlated with involuntary movements (p < 0.05) level. The correlation between the HDRS score and the neurological scores could not be calculated because of the small number of patients with depressive episodes in the sample.
Discriminant analysis was completed for the neuro- logical scores in the sample groups. Discriminant analysis of all scores together was better able to classify between groups than individual scores. Approximately two-thirds of drug-free patients were correctly classified as drug-free and approximately two-thirds of drug-treated patients were correctly classified as drug-treated, whereas all controls were correctly classified. Overall, 75 % of the sample were cor- rectly classified.
Discussion
The main objective of this study was to determine the effect of medication on neurological signs in patients with bipolar affective disorder. In the present study, patients with bipolar affective disorder were found to have higher neurological scores than normal controls, who did not score on any of the items of the neurological battery. A similar excess of neurological signs in patients with bipolar affective disorder has been observed in previous studies.12-16,27-29 The presence of neurological abnormalities in patients with bipolar disorder not currently on drug treatment indicates that the abnormalities are not merely an epiphenomenon of treatment.
It has been argued previously that specific neurological signs could be linked to medication effects.6 In the present study, significantly higher scores in terms of hard signs and involuntary movements were found in the patient group receiving drug treatment, compared with patients not tak- ing medication, indicating that these neurological features might be an effect of medication. The items in the involun- tary movement subscales (intention tremor, choreiform and athetoid movements, postural and resting tremor), occur com- monly as side effects of the drugs, and appear to explain the excess of involuntary movement scores in the drug-treated patients.
A significant positive correlation between scores on hard signs and involuntary movements, and the neurological subscore on the UKU scale in the drug-treated patients further indicates that drugs might be partially responsible for the presence of neurological abnormalities. A similar excess of neurological signs in drug-treated patients has been observed in earlier studies.11,13,18 However, the presence of neurological signs in patients not currently on medica- tion regimens could support the presence of neurological abnormalities in bipolar affective disorder independent of medication effects.
In the drug-treated groups, no significant differences in neurological signs were seen between patients receiving different medications, with the exception of the sensory domain score. A significantly higher score was found in the patients receiving mood stabilisers only. However, as there were only 3 patients in the mood stabiliser-only group, this finding may be spurious.
The significant positive correlation of neurological scores including total score, filtered score, soft signs, motor co- ordination and cognitive domain with the YMRS score implies that neurological abnormalities increase with the severity of mania. This is in keeping with findings reported by Basu et al16 The involuntary movements subscore corre- lated negatively with the YMRS score, suggesting that these test items are not associated with severity of mania, and may represent medication side effects.
In the present study, no significant association was found between age and neurological signs, in agreement with a number of previous studies,15,30,31 but contrasting with Torrey,32 who reported a general association between neurological impairment and increasing age. No significant relationship was found between neurological scores and sex or socio- economic status. This is in contrast to some studies in which significant differences were found according to these demo- graphic variables.11,28,33,34 In the present study, the patients who had less than 10 years of formal education had signifi- cantly higher neurological abnormalities scores than those with a higher educational level. It could be argued that lower education is a proxy index of poor socioeconomic status which can make individuals more prone to insults such as infections and deficiencies during early childhood.34 This seems plausible, at least in developing countries.35 In this study, it was found that residents of rural areas also had significantly higher neurological scores than the residents of urban areas, which could be explained by extending the above argument.
The reported presence of neurological signs in patients with bipolar affective disorder who have not been exposed to psychotropic medication is thought to reflect attentional deficits.31 Basu et al16 reported that performance on the rhythm tapping test and the go-no-go test, measures of attention, concentration and alertness, were impaired in majority of patients during a manic episode and had im- proved significantly during a second assessment four weeks later. Attention, concentration and alertness are known to be impaired during the height of a manic episode.36 In the present study, cognitive items of the neurological assess- ment instrument, gaze persistence and imaginary acts were impaired in the majority of the drug-free patients compared with the drug-treated group. The drug-free and drug-treated groups differed significantly on YMRS scoring, which points to the reduction of symptoms with treatment.
Another point of discussion regarding the neurological signs is the so-called state versus trait effect. McKay et al37 reported enduring neuropsychological deficits in patients with bipolar affective disorder, suggesting a trait effect, at least in a subgroup of patients. Basu et al16 in contrast, reported a decrease in neurological signs in manic patients with resolution of the manic episode.
In addition to a relatively small sample size, a limitation of this study was that the sample consisted predominantly of patients with mania. Patients with depressive episodes were under-represented. Furthermore, the neurological assessment battery was administered by one rater who was blind to neither the diagnosis nor the medication status of the patients.
There is wide variation in the items included as neuro- logical abnormalities in the neurological assessment instru- ments used by previous studies, making comparison across studies difficult. The results of the discriminant analysis showed that the neurological assessment scale was poor at classifying patient groups, though it did correctly identify controls. There is a need for a universally accepted, struc- tured and reliable procedure for rating neurological signs in bipolar affective disorder to make comparison across stud- ies meaningful. The ideal procedure would not include items known to be associated with medication, such as tremor and other abnormal involuntary movements.
Despite methodological limitations, the study adds to the evidence for a higher rate of neurological abnormalities in bipolar disorder, which is consistent and compelling. These abnormalities do not appear to be random but rather are concentrated in motor, cognitive and sensory domains. These findings point toward deficits in the frontal and pari- etal lobe, in contrast to the report by Cherian and Kuruvilla,14 who found that most soft signs in patients with bipolar disorder are related to temporal and parietal lobe function. The left abnormality scores were higher than the right abnormality scores in both the drug-free and drug-treated patients, suggesting a laterality effect. Similar results were reported by Niethammer et al,38 in contrast to higher right- sided scores observed in other studies.32,39 The accumulating body of data make it difficult to interpret the neurological abnormalities as simply representing nonspecific indicators of organic brain dysfunction. In contrast, the neurological abnormalities appear to reflect pathological changes in neural systems that are responsible not only for balance, motor coordination and sequencing, and sensory integration, but also seem to have a vital role in (ab)normal cognition and behavior.40
A further reason for studying neurological abnormali- ties in patients with bipolar disorder is that these patients may have a distinct variant of illness and may be good candidates for valproate therapy.41 The presence of neuro- logical abnormalities has also been noted to be associated with a higher relapse rate during follow-up.13 Compared to other techniques for evaluating neurological aspects of bipolar disorder, the study of neurological signs is inexpensive and can be readily employed with large samples. Further studies are required using neurological assessment batteries along with neuroimaging studies to support exist- ing findings on the relationship between bipolar affective disorder and neurological abnormalities.
References
- Soares JC, Innis RB. Brain imaging findings in bipolar disorder. In:
- Soares JC, Gershon S, editors. Bipolar disorders. New York: Marcel Dekker;2000:227-252.
- Coffman JA, Bornstein RA, Olson SC, Schwarzkopf SB, Nasrallah HA. Cognitive impairment and cerebral structure by MRI in bipolar disorder. Biol Psychiatry 1990;27:1188-1196.
- Videbech P. MRI findings in patients with affective disorder: a meta- analysis. Acta Psychiatr Scand 1997;96:157-168.
- Shioiri T, Oshitani Y, Kato T, et al. Prevalence of cavum septum pellucidum detected by MRI in patients with bipolar disorder, major depression and schizophrenia. Psychol Med 1996;26:431-434.
- Clements S, Peters J. Minimal brain dysfunction in the school-age child. Diagnosis and treatment. Arch Gen Psychiatry 1962;6:185-197.
- Quitkin F, Rifkin A, Klein DF. Neurological soft signs in schizophre- nia and character disorders. Arch Gen Psychiatry 1976;33:845-853.
- Grant IM, Miller M, Reital RW. A neurological study of polydrug users. Arch Gen Psychiatry 1976;33:973-978.
- Gardner D, Lucas PB, Cowdry RW. Soft sign neurological abnormali- ties in borderline personality disorder and normal control subjects. J Nerv Ment Dis 1987;175:177-180.
- Hollander E, Schiffman E, Cohen B, et al. Signs of central nervous system dysfunction in obsessive-compulsive disorder. Arch Gen Psychiatry 1990;47:27-32.
- Gur RE. Motoric laterality imbalance in schizophrenia. Arch Gen Psychiatry 1977;34:33-37.
- Gupta S, Andreasen NC, Arndt S, et al. Neurological soft signs in neu- roleptic-naïve and neuroleptic-treated schizophrenic patients and in normal comparison subjects. Am J Psychiatry 1995;152:191-196.
- Nasrallah HA, Tippin J, McCally-Whitters M. Neurological soft signs in manic patients. A comparison with schizophrenic and control groups. J Affect Disord 1983;5:45-50.
- Mukherjee S, Shukla S, Rosen A. Neurological abnormalities in patients with bipolar disorder. Biol Psychiatry 1984;19:337-345.
- Cherian A, Kuruvilla K. Prevalence of neurological "soft signs" in affective disorder and their correlation with response to treatment. Indian J Psychiatry 1989;31:224-229.
- Negash A, Kebede D, Alem A, et al. Neurological soft signs in bipolar I disorder patients. J Affect Disord 2004;80:221-230.
- Basu S, Ram D, Das SC, Gupta SC. A case controlled study of neuro- logical soft signs in childhood and adolescent mania. Hong Kong J Psychiatry 2002;12:6-10.
- Heinrichs DW, Buchanan RW. Significance and meaning of neuro- logical signs in schizophrenia. Am J Psychiatry 1988;145:11-18.
- King DJ, Wilson A, Cooper SJ, Waddington JL. The clinical correlates of neurological soft signs in chronic schizophrenia. Br J Psychiatry 1991;158:770-775.
- Dazzan P, Murray RM. Neurological soft signs in first-episode psychosis: a systematic review. Br J Psychiatry 2002;181:50-57.
- Wong AH, Vorugonti LN, Heslegraue RJ, Awad AG. Neurocognitive deficits and neurological signs in schizophrenia. Schizophr Res 1997; 23:139-146.
- Donaldson S, Goldstein LH, Landau S, Raymont V, Frangou S. The Maudsley Bipolar Disorder Project: the effect of medication, family history, and duration of illness on IQ and memory in bipolar I disorder. J Clin Psychiatry 2003;64:86-93.
- Young RC, Biggs JT, Ziegler VE, Mayer DA. A rating scale for mania: reliability validity and sensitivity. Br J Psychiatry 1978;133:429-435. 23. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56-62.
- Shamsunder C, Sriram TG, Muraliraj SG, Shanmugham V. Validity of a short 5-item version of the general health questionnaire. Indian J Psychiatry 1986;28:217-219.
- Ismail B, Cantor-Graae E, Cordenal S, McNeil TF. Neurological ab- normalities in schizophrenia: clinical, etiological and demographic correlates. Schizophr Res 1998;30:229- 238.
- Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. The UKU side effect rating scale: a new comprehensive rating scale for psycho- tropic drugs and a cross-sectional study of side effects in neuroleptic- treated patients. Acta Psychiatr Scand 1987;334 (Suppl):1-100.
- Cox SM, Ludwig AM. Neurological soft signs and psychopathology. Incidence in diagnostic groups. Can J Psychiatry 1979;24:668-673.
- Manschreck TC, Ames D. Neurologic features and psychopathology. Biol Psychiatry 1984;19:703-713.
- Woods BT, Kinney DK, Yergelun-Todd D. Neurologic abnormalities in schizophrenic patients and their families: Comparison of schizophrenic, bipolar, and substance abuse patients and normal controls. Arch Gen Psychiatry 1986;43:657-663.
- Kolakowska T, Williams AO, Jambor K. Schizophrenia with good and poor outcome: Neurological "soft signs", cognitive impairment and their clinical significance. Br J Psychiatry 1985;146:348-357.
- Lane A, Colgan K, Moynihan F, et al. Schizophrenia and neurological soft signs: Gender differences in clinical correlates and antecedent factors. Psychiatry Res 1996;64:105-114.
- Torrey EF. Neurological abnormalities in schizophrenic patients. Biol Psychiatry 1980;15:381-388.
- Rockford JM, Detre T, Tucker GJ, Harrow M. Neuropsychological im- pairment in functional psychiatric disease. Arch Gen Psychiatry 1970; 22:114-119.
- Shaji KS, Richard J, Verghese A. Neurologic abnormalities in schizo- phrenic patients and their relatives. Indian J Psychiatry 1990;32: 223-228.
- Gureje O. Neurological soft signs in Nigerian schizophrenics: a con- trolled study. Acta Psychiatr Scand 1988;78:505-509.
- Braden W, Ho CK. Racing thoughts in psychiatric inpatients. Arch Gen Psychiatry 1981;38:71-75.
- McKay AP, Tarbuck AF, Shapleske J, McKenna PJ. Neuropsychologi- cal function in manic-depressive psychosis. Evidence for persistent deficits in patients with chronic, severe illness. Br J Psychiatry 1995; 167:51-57.
- Niethammer R, Weisbrod M, Schiesser S, et al. Genetic influence on laterality in schizophrenia? A twin study of neurological soft signs. Am J Psychiatry 2000;157:272-274.
- Caligiuri MP, Lohr JB. A disturbance in the control of muscle force in neuroleptic-naive schizophrenic patients. Biol Psychiatry 1994;34: 104-111.
- Flashman LA, Flaum M, Gupta S, Andeasen NC. Soft signs and neu- ropsychological performance in schizophrenia. Am J Psychiatry 1996; 153:526-532.
- Stoll AL, Banov M, Kolbrener M, et al. Neurologic factors predict a favorable valproate response in bipolar and schizoaffective disorders. J Clin Psychopharmacol 1994;14:311-313.