Hong Kong Journal of Psychiatry (1999) 9 (2) 13-19
ORIGINAL ARTICLE
ABSTRACT
Objectives: Cerebrolysin is a nootropic drug that directly affects cerebral neurons due to its unique neurotrophic activities. This study evaluated the efficacy and safety of cerebrolysin in patients with mild to moderately severe vascular dementia.
Methods: The trial was a multicentre, double-blind, parallel-group design. 148 patients aged 55 to 85 years were randomised to treatment with either cerebrolysin 30ml or placebo intravenous infusion once daily from Monday to Friday for four weeks. The results were assessed using the Mini-Mental State Examination, the Clinical Global Impression, the Sandoz Clinical Assessment – Geriatric , the Hamilton Depression Scale, the Nuremberg Activities Inventory, the Activities of Daily Living Scale and the modified Trail Making Test.
Results: Patients treated with cerebrolysin showed statistically significant improvement in Mini-Mental State Examination scores and the modified Trail Making Test at the end of treatment compared with patients treated with placebo. The Mini- Mental Health State Examination score was raised by 2.7 points from baseline in patients treated with cerebrolysin. The incidence of treatment-related adverse events with cerebrolysin was low (5%) and comparable to that observed with placebo (8%).
Conclusion: Cerebrolysin leads to fast and clinically relevant improvement in some areas of cognitive function in patients with vascular dementia. There were no significant side effects observed during cerebrolysin treatment.
Keywords: Cerebrolysin; Efficacy; Vascular Dementia
INTRODUCTION
Vascular dementia is due to the effects of one or more cerebrovascular accidents (CVAs) on cognitive function. Typically, vascular dementia is characterised by an abrupt onset and stepwise course, documented by history, focal neurological signs and symptoms, and/or imaging studies. The pattern of cognitive deficits is often patchy, depending on which regions of the brain have been destroyed. Certain cognitive functions may be affected early, whereas others remain relatively unimpaired.
Vascular dementia is the second most common cause of adult dementia after Alzheimer's disease. Different criteria for inclusion account for reported rates of vascular dementia varying from 4.5% to 39% (Jellinger et al. , 1994). As in Alzheimer's disease, the incidence of dementia with evidence of cerebral infarcts increases with age. Vascular disease can damage cerebral tissue through large or small vessel arteriosclerosis, embolus, vasculitis, amyloid angiopathy, or intracranial haemorrhage. In recent years, technical advances in imaging have improved the delineation of cerebral pathology in life and allowed a more precise understanding of the variety of different clinical syndromes that reflect vascular damage to the brain (Sadavoy et al. , 1996). These clinical syndromes include: (1) multi-infarct dementia; and (2) strategic infarct dementia (from a strategically important area such as the angular gyrus or the thalamus); and lesions in the white matter (lacunar infarcts, white matter ischaemia, Binswanger's encephalitis). The term 'multi-infarct dementia' implies a large number of small areas of infarction of the grey matter within the cortex, usually from disease of extracerebral vessels, and the clinical picture reflects the cortical damage with dysphasia, dispraxia and agnosia. In strategic infarct dementia, other clinical features are characteristic of their location such as fluent dysphasia in angular gyrus syndrome. Lacunar infarcts are defined pathologically as a complete cavicating infarct not exceeding 10 mm in diameter in the brain stem, central grey nuclei and the white matter, particularly of the frontal lobes (Jacoby et al., 1997). White matter ischaemia refers to a variety of pathological changes, including incomplete infarctions resulting from narrowing of the intracerebral penetrating arterioles.
Prevention of vascular dementia, or at least a reduction in incidence, may be attainable if appropriate attention is paid to cardiovascular disease before the overt cerebral insult occurs. Currently, there are no specific treatments for the cognitive symptoms of patients with vascular dementia. The medical management of vascular dementia can be expected to reduce or stop the influence of some risk factors or aetiologic elements. Meyer et al., (1986) reported limited improvement with reference to risk factors in patients with vascular dementia; cognition was optimal in patients with hypertension if the systolic blood pressure was controlled, and cessation of smoking benefited nonhypertensive patients. Medications for other somatic disorders may have less predictable effects in patients with vascular dementia compared with elderly or younger patients with dementia of other types. A mixture of ergoloid mesylates, known as hydergine, is marketed for the treatment of nonspecific cognitive impairment. It has been available for at least 40 years and has been studied in at least 150 clinical trials. A recent meta-analysis suggested that there might have been improvements in some neuropsychological and behavioral measures, but the overall effect was not statistically significant. There was a general impression that any improvement observed was in behavioural rather than cognitive measures. In seven trials involving a total of 140 patients with vascular dementia, there was somewhat more compelling evidence of modest improvements in neuropsychological and behavioural measures. A number of additional medications marketed for other indications have been proposed for treatment of vascular dementia such as vitamin E, which has been shown to slow nerve cell damage and death in animal models and cell culture, oestrogen, the hormone melatonin, the calcium blocker nimodipine, and botanical agents such as ginkgo biloba. But these medications have not been subject to sufficient efficacy evaluations (APA, 1997).
Cerebrolysin@ is a nootropic drug containing biologically active peptides. It consists of approximately 15% peptides with a molecular weight not exceeding 10 kD and 85% amino acids based on the total nitrogen. The solution, ready for injection or infusion, is free of proteins, lipids, and antigenic properties. Cerebrolysin 1 ml contains 215.2 mg of porcine brain-derived peptide preparation (cerebrolysin concentrate) in aqueous solution. The peptides act inside the brain at multiple levels. They regulate neuronal energy metabolism, influence behaviour by neuromodulation, and protect nerve cells through neurotrophic stimulation. The effects of cerebrolysin are brain-specific and depend on the peptide fraction of the drug (Windisch et a 1., 1992). Cerebrolysin has regulatory effects on nerve cell metabolism (Piswanger et al., 1990). It influences synaptic plasticity and transmission (Baskys et al. , 1992). Cerebrolysin also improves learning and behaviour in animal experiments. Cerebrolysin reduces the formation of cerebral oedema after bilateral carotid artery occlusion in animal experiments (Kofler et al. , 1989). It can compensate for deficits in spatial orientation in rats. Akai et al., (1992) showed in experiments with transsection of fimbria-fornix that peripheral chronic application of cerebrolysin is able to rescue more than 60% of the cholinergic neurones in the medial septa! nucleus. This protective effect is highly significant. In healthy volunteers, as well as in animal experiments, cerebrolysin has measurable effects on cerebral function and electroencephalogram (EEG) [Trojanova et al. , 1975; Windisch et al., 1985].
The preclinical findings of cerebrolysin led to a number of clinical trials which showed the efficacy of cerebrolysin in neurological diseases (Trojanova et al. , 1975; Windisch et a /. , 1985; Albrecht et al. , 1992; Kofler et al., 1990). There are currently 11 placebo-controlled double-blind trials and 36 clinical trials, including more than 2800 documented patients. Our study group also decided to conduct a clinical trial of cerebrolysin in patients with vascular dementia. The aim of this trial was to determine whether or not cerebrolysin is able to improve the clinical symptomatology and the cognitive performance of patients with vascular dementia.
METHODS
SUBJECTS
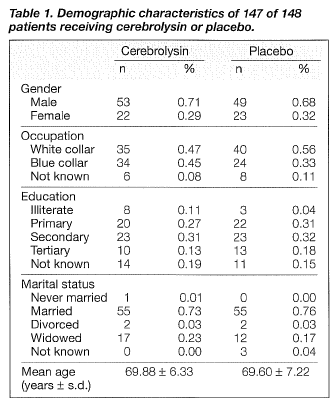
The study included 148 patients aged between 55 and 85 years in whom an established diagnosis of mild to moderately severe vascular dementia according to Diagnostic and Statistical Manual of Mental Disorders- (DSM-) IV criteria (APA, 1994) had been made prior to entry (table 1). In all cases, the presence of Alzheimer's disease was supported by evidence from computed tomographic or magnetic resonance imaging studies performed prior to the study . To be eligible for inclusion in the trial, patients were required to have a Mini-Mental State Examination (MMSE, Folstein et al. , 1975) score between 15 and 25 inclusive, a Hachinski score (HIS, Hachinski et al., 1975) of ?.7, a Global Deterioration Scale (GOS, Reisberg et al., 1982) rating of 3 to 5, and a Hamilton Depression Scale (HAMD, Hamilton, 1960) rating of :s;15. All patients had vision and hearing sufficient for compliance with testing procedures. Female patients were either post-menopausal or had been surgically sterilised prior to entry.
Patients with evidence of other psychiatric or neurological disorders and those who had had clinically significant or active renal, hepatic, endocrine, or cardiovascular diseases were excluded from the trial. The protocol also excluded patients with severe heart disease, severe hypertension, and those with severe obstructive pulmonary disease, haematological or oncological disorders, or vitamin B12 or folate deficiency. None of the patients had a history of alcohol or drug abuse, and none had received investigative drugs within four months of entering the trial. Patients receiving other nootropic agents (unless discontinued at least four weeks prior to the trial), psychotropic drugs, hypnotics (except short-acting benzodiazepines), drugs influencing cerebral blood flow, or stimulants were also excluded.
The study was conducted in accordance with the Declaration of Helsinki. Before initiation of the screening activities, the nature and purpose of the investigation was explained and oral informed consent was obtained from both the patients and their legal representatives.
STUDY DESIGN
The trial was of a multicentre, double-blind, placebo-controlled, parallel-group design. Eligible patients were randomised to one of two treatment groups to receive either cerebrolysin or placebo by intravenous infusion once daily from Monday to Friday for four weeks. The patients were assessed at baseline, and two and four weeks after the start of treatment.
EFFICACY PARAMETERS
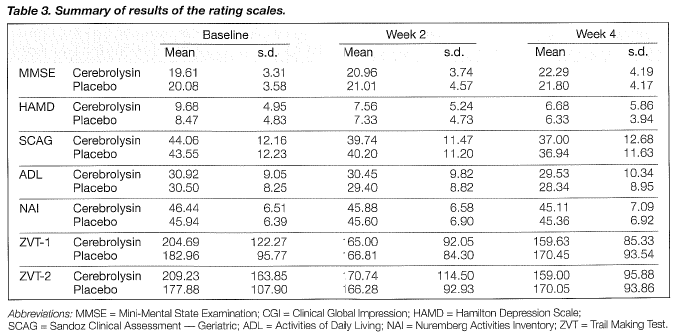
The primary efficacy parameters were change from baseline score of the MMSE and the endpoint rating of the investigator's Clinical Global Impression (CGI, NIMH, 1986). The secondary efficacy measures examined change from baseline scores for the following assessments: the Trail Making Test (ZVT, Oswald et al., 1986), Sandoz Clinical Assessment - Geriatric (SCAG, Shader et al. , 1974), the Activities of Daily Living (AOL, Katz et al. , 1963) rated by the physician and the self-reported Nuremberg Activities Inventory (NA!, Oswald et al. , 1992).
The inter-rater and inter-centre reliability of the MMSE, HAMD, SCAG, and AOL were assessed during the study meetings. The overall inte1viewer-observer reliability coefficient for these scales was greater than 0.85 (range, 0.72 - 0.93).
SAFETY
Adverse events were monitored by scheduled questioning. All events reported by the patients, or noticed by the caregivers or physicians, were recorded together with the date of onset and cessation, severity, and relationship to trial medication on standard international forms based on the American National Adverse Drug Reaction Directory. Haematology was assessed by measurement of haemoglobin , haematocrit erythrocyte count, and total and differential leucocyte and platelet counts. Clinical chemistry parameters were monitored to assess liver function (alkaline phosphates, aspartate transaminase, alanine transaminase, total bilirubin), renal function (blood urea, nitrogen, creatine), metabolic function (total cholesterol, glucose, total protein and albumin), and electrolytes (sodium, potassium, chloride, calcium, phosphorus). Electrocardiogram and routine urinalysis (specific gravity, dipstick chemistry, pH, protein, glucose, ketones, blood) were also performed at baseline and endpoint. Vitamin B12 and folate levels were assessed at screening only. Vital signs assessments included supine and standing blood pressure, heart rate, body temperature, respiratory rate, and body weight.
MEDICATION
The treated group received cerebrolysin 30 ml in 100 ml physiological saline intravenous infusion. The placebo group received placebo 30 ml in 100 ml physiological saline intravenous infusion. The infusions were given daily from Monday to Friday for four weeks. The yellow opaque infusion bottles containing cerebrolysin or placebo (prepared by EBWE Pharmaceuticals, Unterach, Austria) were used to secure the blinding of the trial.
STATISTICAL ANALYSIS
The efficacy conclusions were based on the combined results at each patient's last assessment during double-blind therapy -defined as the study endpoint. All hypothesis tests were two-sided, and p values of ::::0.05 were considered to be statistically significant. Continuous demographic variables (e.g. age, height, weight) were examined using the students t-test and categorical demographic variables were analysed using chi-square test or Fisher's exact test. The ordinal scaled data or data with multi-model distribution were subject to the Wilcoxon-Mann-Whitney U test. Analysis of adverse events was done by tabulating treatment-emergent signs and symptoms, which were defined as events that occurred during or after administration of the first dose of study medication, or became more severe during treatment. The incidence of treatment-emergent adverse events and treatment-emergent laboratory abnormalities were compared across treatment groups by means of Fisher's exact test.
RESULTS
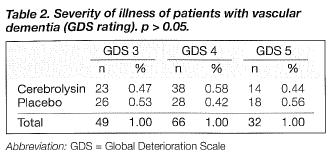
The demographic data for 147of the 148 patients randomised to treatment are shown in table 1. The patients' ages ranged from 55 to 85 years; the mean ages for the patients in the cerebrolysin and placebo groups were 69.88 ± 6.33 and 69.60 ± 7.22, respectively. There were no significant differences between the two groups concerning gender, age, education, and marital status. The severity of dementia for the two groups was comparable according to the GDS rating, with no statistically significant difference (Table 2). One patient, who was excluded from the data analysis, failed to complete the trial due to refusal of treatment.
PRIMARY EFFICACY PARAMETERS
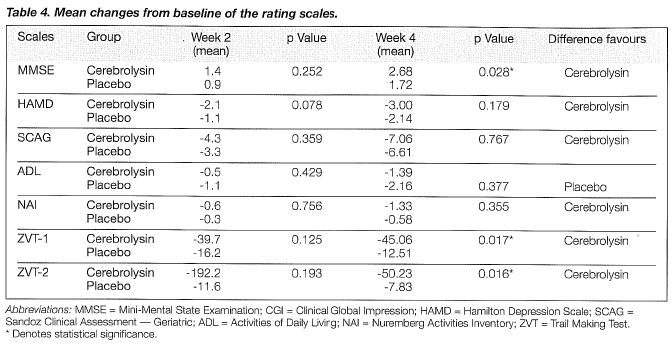
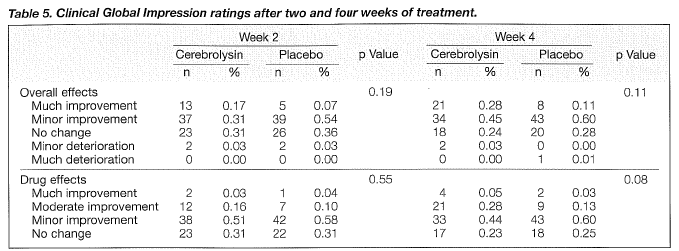
The primary criteria for the evaluation of efficacy were mean changes in the MMSE and CGI. Tables 3 and 4 show the changes in the rating scales. Improvements in the MMSE became statistically significant after four weeks. The mean total scores of MMSE were raised by 2. 7 points for the cerebrolysin group and by 1.7 for the placebo group at the endpoint (Tables 3 and 4, Figure 1). Changes in CGI on the overall effects were not statistically significant between the two groups after four weeks of treatment (Table 5). After four weeks, the ratings of CGI item 2 on drug effects showed a trend towards significance (Table 5, Figure 2).


SECONDARY EFFICACY PARAMETERS
The differences in mean changes of the ZVT tests between the two groups showed statistical significance after 4 weeks in favour of cerebrolysin (Table 4, Figures 3 and 4).
No statistically significant differences in the mean scores of HAMD, SCAG, ADL and NAI were found between the two groups at the endpoint (Table 4).
SAFETY
Cerebrolysin was well tolerated. No severe adverse effects were observed in either group. Regular blood pressure and heart rate monitming did not reveal any drug-relevant changes. There were no clinically relevant changes in laboratory parameters. The incidence of treatment-related adverse events with cerebrolysin (5%) was comparable to that observed with placebo (8%). As shown in Table 6, the most frequently encountered adverse events with cerebrolysin compared with placebo were dizziness (2% us l%, respectively). The majority of treatment-related events were of mild to moderate intensity and in most case, there was no apparent relationship to cerebrolysin.
DISCUSSION
The results presented here demonstrate that cerebrolysin is effective in the treatment of patients with mild to moderately severe vascular dementia. Cerebrolysin provided a statistically significant enhancement in cognitive function as assessed by improvements in MMSE scores. The improvements in MMSE scores for the cerebrolysin group was statistically significantly greater than those observed in patients receiving placebo (Table 4). The overall beneficial effect of cerebrolysin was supported by the improvement for the ZVT tests, which evaluate attention, flexibility, and executive function.
Early in 1986, positive effects of cerebrolysin were found in a study of subjects with degnerative dementia conducted by Hebenstreit et al. (1986). The investigators found cerebrolysin 30 ml to be more effective than the 10 ml dosage. There were no changes in a self-designed global clinical assessment score, but statistically significant differences were seen in reaction time and EEG changes. Kofler et al. (1989, 1990) examined the nootrophic effects of cerebrolysin in patients with organic brain syndrome. One-third of the patients had primary degenerative dementia and two-thirds suffered from vascular dementia. Cerebrolysin 20 ml was administered daily for 10 days. Statistically significant improvement in the cerebrolysin group was observed in the SCAG, self-assessment, cognitive performance in the NAI, the syndrome short test, and the concentration test for the elderly. It is interesting that these findings were confirmed with electro-physiological methods. Vereshchagin et al. (1991) found a positive effect in the clinical symptomatology in patients with vascular dementia. There was a significant improvement in abstract thinking and memory performance. Recently, a further study conducted by Ruther et al. (1994) demonstrated that cerebrolysin leads to a significant, fast, and relevant improvement in the clinical symptoms of patients with Alzheimer's disease. The cerebrolysin group showed efficacy on all levels of observation. All the above-mentioned studies were double-blind, placebo-controlled trials. A uniform positive effect on higher brain functions was observed. The efficacy in Ruther's study seems better than that of our study and all efficacy measures showed statistically significant improvement for patients receiving cerebrolysin. However, this study did not use the efficacy measurement of MMSE, which is widely accepted, and the patients included had more mild disease than those in our study (with patients rated as GDS 5 being excluded). It should be emphasised that other agents such as tacrine and donepezil, which are proved to be effective for patients with mild to moderate Alzheimer's disease, usually take a long time (3 - 6 months) to show efficacy (APA, 1997). The efficacy of cerebrolysin in treating dementia of various aetiologies is probably due to its complex composition. Cerebrolysin is a nootrophic drug consisting of free amino acids and peptides. It has been demonstrated that the effects of cerebrolysin are brain specific, and cerebrolysin can exert a regulatory effect on neuronal metablism. It influences synaptic plasticity and transmission, and improves learning behaviour in experimental models. The neurotrophic action of cerebrolysin is not only observed in cells of the peripheral nervous system but also in cells of the central nervous system. The nootrophic peptides may have the ability to cross the blood-brain barrier. Akai et a I. (1992) demonstrated that cerebrolysin protects the cholinergic neurones of the medial septum after damage to the fimbriafornix. Recently, Xiong et al. (1995) found that cerebrolysin depressed synaptic transmission at the commissural pathway in the CAl area of rats. Detailed analysis showed that the inhibition is presynaptic and can be reduced by low doses of a specific blocker of adenosine Al receptors. Therefore, cerebrolysin may indirectly suppress glutamatergic synaptic transmission and possibly act as a brain endogenous neuroprotective agent. During the past two decades, many papers have been published and have confirmed the neurotrophic effects of this peptidergic drug in vitro and in vivo. These experimental findings strongly indicate that cerebrolysin has a possible protective mechanism for neurones and enhances the function of surviving neurones after impairment.
The safety profile of cerebrolysin at the dosage studied in this trial was similar to that of placebo with regard to changes in vital signs, the incidence of adverse events, or the occurrence of clinical laboratory abnormalities.
In conclusion, this study indicates that cerebrolysin at a dosage of 30 ml once daily for 4 weeks provides significant improvements in some areas of cognitive function in patients with vascular dementia. Cerebrolysin administration is not associated with or complicated by severe adverse events.
REFERENCES
Akai F, Hiruma S, Sato T, et al. Neurotrophic factor-like effect of 1070 on septa! cholinergic neurones after transections of fimbriafornix in the rat brain. Histol Histopathol 1992;7:213-221.
Albrecht E, Hinge[ S. The effects of cerebrolysin on the vitality and sprouting of neurons from cerebral hemispheres and from the brain stem of chicken embryos in vitro. Neurobiol Aging 1992;13 (Suppl. l):127.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association, 1994.
American Psychiatric Association. Practice guidelines for the treatment of patients with Alzheimer's disease and other dementias of late life. Am J Psychiatry 1997;5 (Suppl.):1-39.
Baskys A, Wojtowicz JN. Actions of organ derived preparations on synaptic transmission in the hippocampus. Neurobiol Aging 1992;13 (Suppl. 1):128.
Folstei n MF, Folstein SE, Huge BK, et al. Mini Mental State Examination: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:1989-1998.
Hachinski VC, Iliff LO, Zilhka E, et al. Cerebral blood flood flow in dementia. Arch Neural 1975;32:632-637.
Hamilton M. A rating scale for depression. J Neural Neurosurg Psychiatry 1960;23:56-62.
Hebenstreit GF. Die wirkung eines aminosature-peptid-extraktes beizerebralen f unktionsstorrungen in geronton psychitrie. Neuropsychiatre 1986;1:38-44.
Jacoby R, Oppenheimer C. Psychiatry in the Elderly. 2nd ed. Oxford: Oxford Medical Publications, 1997.
Jellinger KA, Ladurner G, Windish M. New trends in the diagnosis and therapy of Alzheimer's disease. New York: Springer-Verlag, 1994.
Katz S, Ford AB, Moskowitz RW, et al. Studies of illness in aged: the index of AOL, a standardized measure of biological and psychosocial funtion. J Am Med Assoc 1963;185:94-106.
Kofler B, Erhart C, Erhart P, et al. About the therapeutic efficacy of cerebrolysin. Psycho 1989;8:29-33.
Kofler B, Erhart C, Erhart P, et a /. A multidimensional approach in testing nootripic drug effects (cerebrolysin). Arch Gerontol Geriatr 1990;10: 129-140.
Meyer JS, Judd BW, Tawakina T, et al. Improved cognition after control of risk factors for multi-infarct dementia. J Am Med Assoc 1986;256:2203-2209.
National Institute of Mental Health: Clinical Global Impression. In: CIPS - Collegium Internationale Psychiatriae Scalarum, eds. Internationale skalen fur psychiatrie. Washington: Beltz Test, 1986. Oswald WO. Der Zahlen-Verbindungs-Test im hoheren lebensalter. In: Aspekte psychologischer porschung. Gottingen: Hogrefe, 1986; 377-388.
Oswald WO, Fleischmann UM. Nurenberger-Alters-lnventar NAI. Testkasten und manual. Gottingen: Hogrefe, 1992.
Piswanger A, Paier B, Windisch M. Modulation of protein synthesis in a cell-free system from rat brain by cerebrolysin during development and aging. Amino Acids 1990;3:651-657.
Reisberg B, Ferris SH, De Leon MJ, et al. An instrument for the assessment of primary degenerative dementia (POD). Am J Psychiatry 1982;139:1136-1139.
Ruther E, Ritter R, Apeccechea M, et al. Efficacy of the peptidergic nootropic drug cerebrolysin in patients with senile dementia of Alzheimer type. Pharmacopsychiatry 1994;27:32-40.
Sadavoy J, Lazarus LW, Jarvik LF, et al. Comprehensive Review of geriatric psychiatry. 2nd ed. Washington, DC: APA Press Inc, 1996.
Shader R, Harmatz JS, Salzman C. A new scale for clinical assessment on geriatric populations: Sandoz Clinical Assessment - Geriatric (SCAG). J Am Geriatr Soc 1974;22(3):107-113.
Trojanova M. Influence of cerebrolysin on the resistance of rats to anoxia. Physiologia Bohemoslovaca. 1975;25:319-323.
Vereshchagin N, Lebedeva NV, Suslina ZA, et al. Mild forms of multiinfarct dementia: effectiveness of cerebrolysin. Sowj Med 1991;11:6-8.
Windisch M, Piswanger A. Changes of brain metablism of rats due to long term treatment with a peptide derivative. Drug Res 1985;35:1353-1356.
Windisch M, Albrecht U, Eggenreich B. Neurotrophic effects. Aging 1992;13 (Suppl. 1):133.
Xiong H, Wojtowicz J, Baskys A. Brain tissue hydrolysate acts on presynaptic adenosine receptors in the rat hippocampus. Can J Physiol Pharmacol 1995;73: 1194-1197.
Acknowledgement: This study was sponsored by Ebewe Pharmaceuticals, Unterach, Austria.
Dr S Xiao, MD, Dr H Yan, MD, Dr P Yao, MD, Shanghai Mental Health Center, Shanghai, China.
Dr L Wang, MD, Dr J Jia, MD, No. 301 Hospital of the People's Liberation Army, Beijing, China.
Dr X Ma, MD, Dr F Bao, MD, Beijing An Ding Psychiatric Hospital, Beijing, China.
Dr J Long, MD, Dr C Wang, MD, Beijing Tian Tan Hospital, Beijing, China.
Dr Z Dai, MD, Dr M Zhang, MD, Tianjing Medical University Hospital, Tanjing, China.
Dr W Xu, MD, Gou Yinglu, MD, West China Medical University Hospital, Chengdu, China. Dr C Ma, MD, Dr D Wang, MD, Guang Zhou Psychiatric Hospital, Guangzhou, China.
Dr B Su, PhD, Shanghai Second Medical University, Shanghai, China.
Address for reprints: Dr Xiao Shifu
Shanghai Mental Health Center
600 Wan Ping Nan Road
Shanghai 200030, China.</p


