Hong Kong Journal of Psychiatry (1997) 7 (2) 36-39
SPECIAL TOPIC: SCHIZOPHRENIA
Summary
To investigate auditory P300 variations and their correlation with clinical feature in depressives. The auditory P300 was recorded from 18 unmedicated and 32 medicated depressives and 35 normal controls, using a standard ddball’ paradigm. The P300 latency was delayed in both depression groups as compared to the control ( p<0.01) , and was significantly correlated with the MMSE ( Mini- Mental State Examination) score (p<0.05). A lower P300 amplitude was found in both patient groups (p< 0.05 or P< 0.01). The unmedicated depressives presented lower P300 amplitudes dominantly at sites in the right hemisphere. The P300 latency was suggested to be an electrophysiological indicator reflecting the cognitive dysfunction of depressives. The scalp distribution of lower P300 amplitude over the right hemisphere in the unmedicated group supported the hypothesis of right-hemisphere dysfunction in depression.
Keywords: P300, depression, unmedicated, medicated
INTRODUCTION
P300 component of event-related potentials has been the subject of intense psychophysiological investigation as a marker related to cognition and signifies the integrity of informationprocessing functions of the brain. Evidence suggests that P300 is modulated through noradrenergic, serotoninergic, and dopaminergic systems (Garreau et al., 1984; Wood et al., 1984; Ito et al., 1990).
Very few studies on P300 are available in major depression and the findings of reported studies are far from being consistent, especially when compared with the P300 amplitude attenuation found in schizophrenia. A few studies have found that P300 amplitude is smaller in depressed patients than in normal controls, but larger than in schizophrenic patients (Levit et al., 1973; Shagass et al., 1981; Baribeau-Braun & Lesevre, 1983; Pfefferbawn et al., 1984; Blackwood et al., 1987). An equal nwnber of studies negated this finding and reported that no P300 latency or amplitude differences existed between depressives and normals (Pfefferbawn et al., 1984; Gordon et al., 1986; Their et al., 1986; Patterson et al., 1988; Have et al., 1991). Only one study (Bruder et al., 1991) has reported a significantly prolonged P300 latency in ypical' depressives (melancholics and simple mood reactive depressives); however, subjects in this study performed a spatial discrimination task. At least three major factors may account for these conflicting results: the heterogeneity of samples (different subtypes of depressives, severity of symptoms), the treatment intervention, and the paradigm used to elicit P300.
The purposes of this study were to investigate (1) whether the P300 variables of depressives are different from th of normal subjects, and (2) whether the P300 variables of depressives correlate with their clinical ratings.
METHODOLOGY
SUBJECTS
The subjects were 50 depressed patients (divided into 2 subgroups, 18 unmedicated and 32 medicated) and 35 normal controls, aged 18 to 60. All subjects were screened for current or past history of substance abuse, organic brain impairment, or somatic diseases.
The 35 normal subjects (19 male, 16 female; mean age, 34.6±11.7 years) were volunteers recruited from staff of Shanghai Mental Health Center and students of Shanghai Second Medical University.
The 50 patients were recruited from the Outpatient Department and Inpatient Wards of Shanghai Mental Health Center. The patients were diagnosed to have major depression according to DSM-III-R criteria (American Psychiatric Association, 1987) by at least 2 experienced psychiatrists and none of them had any axis II diagnosis. Patients with a history of electroconvulsive treatment during the 6 months before the study were excluded. For the patient group, 18 (10 male, 8 female; mean±D: 37.9±11.2 years) had been drug free for at least 2 weeks before testing, and the rest (15 male, 17 female; mean±D: 43.4± 12 .1 years) were receiving tricyclic antidepressants (TCAs) (mean±D: 146.1±57.2 mg/day). The patients had received TCAs for about 2 weeks but were not yet relieved of their depression. Most of the patients suffered from unipolar depression, and endogenous features were common (Table 1).
The patients and the control subjects were matched grossly on socioeconomic and demographic variables.
All patients were assessed by the Chinese versions of the Hamilton Rating Scale for Depression (HAMD) (Hamilton, 1967; Zhang, 1993) and the Mini-Mental State Examination
(MMSE) (Folstein, 1975; Zhang 1993). The respective mean and SD of their HAMD and MMSE scores were 26.8±5.6 and 28.2±1.6 (unmedicated group) and 28.8±6.3 and 27.0±2.7 (medicated group).

PROCEDURE
The P300 test was carried out in a soundproof room. Subjects were tested between 9 am and 12 am on the same day as their clinical and cognitive assessments. The subjects were instructed to sit on a comfortable chair, relax, keep vigilant and close their eyes lightly.
The P300 was assessed using Dantec Concerto SEEG-16 BEAM. Platinum disc electrodes of 8 mm diameter were used and bipolar recordings were made at F3, Fz, F4, C3, Cz, C4, P3, Pz and P4, referred to linked mastoid leads. The mid-point of the forehead was grounded.· lnterelectrode impedance was maintained at less than 5 kiloOhms. Eye-movement detection and rejection of contaminated trials was made automatically by the instrument.
A standard "oddball' paradigm was used to elicit the auditory P300. Sounds were delivered biaurally through earphones. The stimulus parameters were preprogrammed and were the same for all subjects. The frequency of stimulus l (S1) was 1000 Hz and that of stimulus 2 (S2) was 2000 Hz. The delivery rate was 0.5/sec with a sound level of 60db (S1) and 85db (S2). The probability of S2:Sl was 1:4. The subject was asked to count S2 silently until 250 artifact-free sweeps were obtained (200 sweeps for S1, and about 50 sweeps for S2), which constituted one trial. The trial was rejected if the subject failed to detect more than 10 S2 events. Responses were stored on disk for further analysis. The mean and SD of errors in the oddball task were 0.29±0.93 for normal controls, 1.39±2.38 for unmedicated and 2.12±3.09 for medicated patients.
ANALYSIS
The P300 component was identified from the averaged event-related potentials waveform as a positive voltage peak 250-600 ms after stimulus presentation. The peak latency and amplitude of P300 were measured for all subjects.
Intergroup differences of the P300 variables were tested using an analysis of variance (ANOVA) and Duncan New Test. Linear correlation was calculated for clinical and cognitive ratings and P300 variables within the patient groups. lnterhemispheric differences of P300 amplitude were revealed by paired t-test.
RESULTS
P300 LATENCY (CZ)
No significant difference of P300 latency was found between different electrode sites. Only the P300 latency at Cz was used for the following analysis.
The mean P300 latency was 301.4±19.1 msec for controls, 327.9±22.0 msec for unmedicated depressives, and 342.6±22.4 msec for medicated depressives. ANOVA revealed a significant difference of P300 latency among these 3 groups (F2,82= 32.91, p<0.01). Duncan's New Test confirmed that the P300 latency was significantly delayed in both patient groups as compared to normal controls (unmedicated vs control, Q=6.15, p<0.01; medicated vs control, Q=ll.34, p<0.01); it was also significantly longer in the medicated than in the unmedicated group (Q=3.36, p<0.05).
No significant correlation was found between P300 latency and the total HAMD score (unmedicated, r=-0.088, p>0.05; medicated, r=-0.265, p>0.05). No HAMD factor scores correlated significantly with P300 latency in both patient group. But there was a significant inverse correlation between patients' P300 latency and their total MMSE scores (unmedicated, r=- 0.513, p<0.05; medicated, r=-0.378, p<0.05).
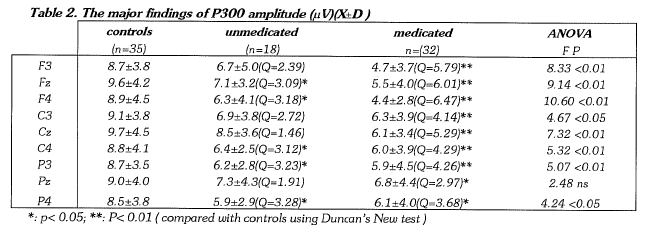
P300 AMPLITUDE & ITS SCALP DISTRIBUTION
ANOVA revealed significant differences of P300 amplitude among the 3 subject groups at all recording sites except Pz. By using Duncan New Test, a significantly lower P300 amplitude was found in the unmedicated group than in normal controls at Fz, F4, C4, P3 and P4 (p<0.05), which was dominantly distributed over the right hemisphere; the medicated group showed a significantly lower P300 amplitude than the controls at all recording sites (F3, Fz, F4, C3, Cz, C4 , P3: p<0.01 ; and Pz, P4: p<0.05).
Differences of P300 amplitude between symmetrical recording sites were tested within each subject group by a paired t-test. A significant asymmetry of P300 amplitude was only found for the F3-F4 comparison within the unmedicated depressives (t= 2.62, p<0.05), who showed a lower P300 amplitude at F4 than at F3 (Table 3).
The P300 amplitudes (e.g. at Cz) of both patient groups were not significantly correlated with the HAMD scores (unmedicated, r=-0.39, p>0.05; medicated, r=-0.23, p>0.05) or the MMSE scores (unmedicated, r=0.082, p>0.05; medicated, r=0.151, p>0.05).

DISCUSSION
The present study demonstrates a delayed P300 latency at Cz in both unmedicated and medicated depressives as compared to normal controls. Up to now, only a few researchers have reported a delayed P300 latency in depressives (Bruder et al 1991), which is in sharp contrast with these patients' clinical feature of reactive slowness. Patterson and coworkers (1988) reported increased P300 latency variability in depressives as compared to controls, suggesting the heterogeneity of depressed subjects might undermine the consistency of the findings reported. After reviewing the history of our 50 depressives, we found that 46 of them were identified as having unipolar depression (the remaining 4 patients had a history of manic episodes, 1case from the unmedicated group, 3 cases from the medicated group ). The relatively consistent history of no manic episodes, combined with the severity of depression (the mean HAMD score was 26.8±5.6 for unmedicated, and 28.8±6.3 for medicated), may underlie the positive P300 latency finding in this study using the standard "oddball' paradigm. Furthermore, the P300 latency was found to be longer in the medicated than in the unmedicated group. Since scopolamine directly prolongs P300 latency in normal subjects by its anticholinergic effect (Callaway et al., 1985), the strongly anticholinergic action of antidepressants, especially the TCAs, could explain the longer latency in the medicated group.
Another major finding of this study was a lower P300 amplitude in both depressive groups than in normal controls. Similar findings have been reported by many researchers (Roth & Cannon, 1972; Levit et al., 1973; Steinhauer & Zubin, 1982; Baribeau-Braun & Lesevre, 1983; Pfefferbaum et al., 1984; Blackwood et al., 1987). We found that medicated depressives presented a lower P300 amplitude at all recording sites, but unmedicated ones only showed this change at 5 sites ( Fz, F4, C4, P3 and P4 ) of which 3 (F4, C4 and P4) were located on the right hemisphere and only 1 (P3) on the left hemisphere. Within the unmedicated group, an asymmetry of P300 amplitude was revealed in the F3-F4 comparison, implying a lower P300 amplitude at F4 than at F3.
When correlating the P300 with these depressives' clinical features, a significant correlation was found only between the P300 latency and one rating of cognitive function (total MMSE score). A large body of literature has elaborated this correlation in patients with dementia (e.g. Goodin et al., 1978, 1983; Polich et al., 1986, 1990). We have extended this relationship to depressives, suggesting the P300 latency may be used as an electrophysiological indicator reflecting depressives' cognitive dysfunction. The cognitive disturbance in depression is often manifested during the acute episode, particularly with respect to aspects of attention, learning and memory, and is viewed as a secondary manifestation of the illness. But there is also evidence that the dysregulation of functional brain systems that gives rise to episodes of affective illness may directly impact on functioning in both the affective and cognitive spheres (Georgotas et al., 1988). The significant correlation of P300 latency with total MMSE score but not with total HAMD score within our sample confirmed the clinical impression that, in depressives, the extent of cognitive dysfunction often does not parallel the degree of depression.
No significant correlation was found between P300 amplitude and these patients' cognitive function or depression, suggesting its context be something different. The lower P300 amplitude has been suggested as a state marker of depression (Blackwood et al., 1987), and recently it was correlated with the psychotic symptoms of depressives (Santosh et al., 1994). The scalp distribution of lower P300 amplitude dominantly over the right hemisphere in unmedicated depressives, first revealed here, provided further evidence supporting the hypothesis of right-hemisphere dysfunction in depression. The symmetrical scalp distribution of lower P300 amplitude in the medicated depressives may be attributed to the intervention of tricyclic antidepressants.
The follow-up study of our sample is ongoing to investigate the biological nature of the above-mentioned P300 changes in depressives whether they are state or trait markers of depression. Future P300 studies will focus on the subtypes of depresion.
ACKNOWLEDGMENTS
Support for this study was provided by Shanghai Second Medical University. The authors are grateful to Dr. I.C. Bruce, Department of Physiology, University of Hong Kong, for his helpful comments on the manuscript.
REFERENCES
American Psychiatric Association (1987) Diagnostic and Statis tical Manual of Mental Disorder. 3rd rev. Washington DC: American Psychiatric Association.
Baribeau-Braun, J., Lesevre, N. (1983) Event-related potentials assessment of psychomotor retardation in depressives. Advances in Biological Psychiatry 13:211-224.
Blackwood, D.H.R., Whalley, L.J., Christie, J.E., Blackburn, l.M., Clair, D.M.T., Mclnnes, A. (1987) Changes in auditory P3 event related potential in schizophrenia and depression. British Journal of Psychiatry 150: 154-160.
Bruder, G.E., et al. (1991) Event-related potentials in depression: influence of task, stimulus hemifields and clinical features on P3 latency. Biological Psychiatry 30: 233-246.
Callaway, E., Halliday, R., Naylor, H., Schechter, G. (1985) Effects of oral scopolamine on human stimulus evaluation. Psycho pharrnacology 85: 133-138.
Folstein, M.F., Folstein, S.E., McHugh, P.R. (1975) "Mini-Mental State". A practical method for -grading the cognitive state of patients for the clinician. Journal of Psychiatry Research 12: 189-198.
Garreau, B., Martineau, J., Barthelemy, C., Lelord, G. (1984) Electrocortical activity ( evoked potentials ) and dopamine metabolism (homovanillic acid) in normal and emotionally disturbed children. Annals of the New York Academy of Sciences 425: 353-356.
Georgotas, A., Cancro, R. (1988) Depression and Mania. Elsevier Science Publishing Co. Inc., New York. pp 265-289.
Goodin, D.S., Squires, K C., Starr, A. (1978) Long latency eventrelated components of the auditory evoked potential in dementia. Brain 101: 635-648.
Goodin, D.S., Starr, A., Chippendale, T., et al. (1983) Sequential changes in the P3 latency component of the auditory evoked potential in confusional state and dementing illness. Neurology 33: 1215-1218.
Gordon, E., Kraiuhin, C., Harris, A., Meares, R., Howson, A. (1986) The differential diagnosis of dementia using P300 latency. Biological Psychiatry 21: 1123-1132.
Hamilton, M. (1967) Development of a psychiatric rating scale for primary depression. British Society of Clinical Psychology 6: 278-296.
Have, G., Kolbeinsson, H., Petursson, H. (1991) Dementia and depression in old age: Psychophysiological aspects. Acta Psy chiatrica Scandinavia 83: 329-333.
Ito, J., Yamao, S., Fukuda, H., Mimori,Y., Nakamura, S. (1990) The P300 event related potentials in dementia of Alzheimer type: correlation between P300 and monoamine metabolites. Electroencephalography & Clinical Neurophysiology 77: 174-178.
Levit, A.L., Sutton, S., Zubin, J. (1973) Evoked potential correlates of information processing in psychiatric patients. Psychological Medicine 3: 737-794.
Patterson, J.V., Michaleski, H.J., Starr, A. (1988) Latency variability of the components of auditory event-related potentials to infrequent stimuli in aging, Alzheimer-type dementia and depression. Electroencephalography & Clinical Neurophysiology 71(6): 450-460.
Pfefferbaum, A., Wenegrat, B.G., Ford, J.M., et al. (1984) Clinical application of the P3 component of event-related potentials. 2. Dementia, depression and schizophrenia. Electroencephalo graphy & Clinical Neurophysiology 59: 104-124.
Polich, J., Ehlers, C.L., Otis, S., et al. (1986) P300 latency reflects the degree of cognitive decline in dementing illness. Electroen cephalography & Clinical Neurophysiology 63: 138-143.
Polich, J., Ladish, C., Bloom, F.E. (1990) P300 in early Alzheimer's disease. Electroencephalography & Clinical Neu rophysiology 77: 179-185.
Roth, W.T., Cannon, E.H. (1972) Some features of the auditory evoked response in schizophrenics. Archives of General Psychiatry 27: 466-471.
Santosh, P.J., Malhotra, S., Raghunathan, M., et al. (1994) A study of P300 in melancholic depression correlation with psychotic features. Biological Psychiatry 35: 474-479.
Shagass, C., Roemer, R.A., Straumanis, J.J., et al. (1981) Differentiation of depressive and schizophrenic psychosis by evoked potentials. Advances in Biological Psychiatry 6: 173-179.
Steinhauer, S., Zubin, J. (1982) Vulnerability to Schizophrenia: Information processing in the pupil and event-related potential. In Usdin, E., Hanin, L. (eds). Biological Markers in Psychia try and Neurology. London: Pergamon Press.
Their, P., Axmann, D., Giedke, H. (1986) Slow brain potentials and psychomotor retardation in depression. Electroencephalography & Clinical Neurophysiology 63: 570-581.
Wood, C.C., Allison, T., Goff , W.R., et al. (1984) Anatomical and physiological substrates of event-related potentila. Annals of the New York Academy of Sciences 425: 696-721.
Zhang MY (1993): A Manual of Rating Scales in Psychiatry. Hunan Science & Technology Publishing House, Hunan. ppl22-127 & 185-188. (Chinese)
* Wang Jijun, Department of Electrophysiology, Shanghai Institute of Mental Health,
Zhang Mingdao, Department of Psychiatry, Shanghai Second Medical University,
Chen Xingshi Department of Electrophysiology, Shanghai Institute of Mental Health,
Lou Feiying Department of Electrophysiology, Shanghai Institute of Mental Health,
Liang Jianhua Department of Electrophysiology, Shanghai Institute of Mental Health,
* Correspondence: *Dr. Wang Jijun, Department of Electrophysiology, Shanghai Institute of Mental Health, 600 Wanping Road, Shanghai 200030, P.R. China


