Hong Kong J Psychiatry 2001;11(4):2-8
ORIGINAL ARTICLE
S Guo, Z Jiang, Y Wu
Acknowledgments: The authors are indebted to the following colleagues for their close collaboration in follow-up of patients and case management: Dr Yang Zheng, Dr Liu Yuebiao, Dr Su Mujin,
Dr Liu Huacheng, Dr Sha Lijun, Dr Zhang Dianmin,
Dr Luo Xiaoyun, Dr Yang Jiewen, Dr Zhuo Fuzhen, Dr Xu Ruerong, Dr Liang Rejun, Dr Li Dongjun, Dr Chen Guoqiang, Dr Ma Cui.
Prof S Guo, MD, Associate Professor of Psychiatry, National Drug Dependence Treatment Centre, Beijing An Ding Hospital, Beijing, China Prof Z Jiang, MD, FRCPsych, Professor of Psychiatry, National Drug Dependence Treatment Centre, Beijing An Ding Hospital, Beijing, China Prof Y Wu, MD, Associate Professor of Psychiatry, National Drug Dependence Treatment Centre, Beijing An Ding Hospital, Beijing, China
Address for correspondence: Prof S Guo, National Drug Dependence
Treatment Centre, Beijing An Ding Hospital, Beijing 100009, China
E-mail: guos@ihw.com.cn
Submitted: 16 October 2000; Accepted: 6 August 2001
Abstract
Objective: To investigate the efficacy of naltrexone in preventing relapse of heroin addiction among Chinese patients after successful detoxif ication.
Methods: 302 heroin addicts were enrolled in a double-blind and an open trial conducted in 7 treatment centres for 6 months. Naltrexone 50 mg/day was given to the patients in the double-blind study and the dose was titrated from 10 to 50 mg/day in the open trial, based on the patients’ responses.
Results: In the double-blind study, 28.57% of naltrexone-treated patients completed the 6-month treatment course, while 7.14% of the group receiving placebo remained abstinent. In the open trial, the abstinence rate at 6 months was 23.6% and the average length of naltrexone treatment was 3.16 months compared with a previous abstinence rate of 1.2% and drug-free period of 0.5 months The non-euphoric effects reported in the naltrexone and placebo groups were 68.18% and 33.3%, respectively. The rate of positive morphine urine tests was 24.38% for the naltrexone-treated group and 40.48% for the placebo recipients. The blocking effect of naltrexone 50 mg/day was more efficient than the medium and low doses given in the open trial.
Conclusion: The results of this study suggest that naltrexone is an effective medication for the prevention of relapse for heroin-dependent patients, with only mild side effects.
Key words: Naltrexone, Heroin, Drug dependence, Prevention of relapse
Introduction
Many narcotic-dependent individuals who detoxify from heroin addiction relapse due to various psychological or environmental precipitating factors. In animal experiments, it is assumed that the euphoric effects of opiates act as a positive reinforcement of the addiction. However, if a narcotic antagonist is given, no euphoria will be experienced after opiate administration. Hence, minimi- sation of reinforcement via systematic opiate antagonist medication should result in the extinction of heroin craving and self-administration in experimental or psychological models.1-3
Naltrexone hydrochloride, a competitive long-acting opiate receptor antagonist, has been used for the prevention of relapse of heroin dependence. By blocking the access of the agonist to the opiate receptor sites, naltrexone blocks analgesia, euphoria, and other physiological changes. Furthermore, naltrexone plays a role in the prevention of opium addiction relapse.4-5
Naltrexone is now used internationally as an adjunctive medication for relapse prevention in opiate addiction. The abstinence rate for 6 months is approximately 25 to 30%, and is higher in some reports in which psychological and social support were available.5-9
The present study was performed to assess the eff icacy of naltrexone in the prevention of relapse of opiate- dependence by maintaining abstinence from opiates after detoxif ication. The adverse effects of naltrexone and correct dosage for Chinese addicts were also assessed. The study was conducted in 7 treatment centres in China and included double-blind and open trials. The number of patients involved was 302 and the study period lasted for 6 months.
Methods
Patients
Diagnostic and Statistical Manual of Mental Disorders (DSM) -IV criteria of opioid dependence. The age range was 16 to 45 years. All patients had a history of relapse, but none had severe physical or mental disease, and the results of laboratory examinations such as X-rays, electro- cardiography, and liver function tests were normal.
Patients had successfully completed detoxif ication without using opiates for at least 7 to 10 days and the results of the urine test for morphine were negative. Patients had to have a strong desire to abstain from opiates and volunteered to accept naltrexone treatment. The patients’ relatives and/ or friends (sponsors) understood the treatment and guaranteed to supervise the patient’s treatment.
Patients were excluded from the study if they were receiving treatment with any kind of opiate, were still dependant on opiates, had acute withdrawal symptoms, showed apparent withdrawal symptoms after the naloxone challenge test, had a positive urine test for morphine, were allergic to naltrexone, or had severe physical or mental disease.
Treatment Schedules
In the double-blind trial, the randomisation was performed according to the tables of random permutation. Due to ethical considerations of Chinese culture, the number of patients given placebo was limited and the ratio of patients receiving naltrexone to those receiving placebo was 2:1. The naltrexone and placebo doses were prepared by the Toxicology and Pharmacology Department at the Institute of Military Medical Sciences and standardised for equivalent colour and taste. The naltrexone dose was available in 5 mg tablets.
The double-blind trial was performed in 3 of the 7 treatment centres (the National Drug Dependence Treatment Center, Guangzhou Mental Hospital, and the Detoxification and Rehabilitation Center of Shenzhen) where patients were randomised to receive naltrexone or placebo. The open trial was performed in the other 4 research centres (No. 307 Hospital of the People’s Liberation Army (PLA) in Beijing, Guang Zhou Sanatorium of the PLA, The General Hospital of the PLA for Cheng Du in Kun Ming, and No.177 Hospital of the PLA in Guangzhou), where patients were entered into the open study groups.
In preparation for naltrexone treatment, a medical history was taken and mental status tests, and physical and labora- tory examinations were performed. The naloxone challenge consisted of naloxone 0.8 mg given intramuscularly and the patient observed for withdrawal syndromes for 45 minutes. Patients’ urine was tested for morphine and the treatment protocol was read to the patients and their sponsors in both studies. The study duration was 6 months.
The medication was administered once a day after breakfast, supervised by the sponsor. The dose of naltrexone in the double-blind study was 50 mg/day according to reports in the international literature. To investigate the optimal dose for Chinese patients, naltrexone was titrated according to the patients’ responses. However, some patients refused to take the higher dose because of the expense of the medication and possible side effects.
To avoid severe withdrawal syndromes and adverse effects that could be precipitated by naltrexone 3 to 5 days were required to titrate the dose of naltrexone from 5 mg a day in the open trial study. Maintenance treatment started after 5 days and the dose of naltrexone was titrated according to the patient’s responses.
In the double-blind study, the testing procedure was the same as for the open study. Patients in both the naltrexone and placebo groups were given the medication in the same way, with the same amount of tablets. Patients in the naltrexone group received naltrexone 50 mg/day (10 tablets) in the morning.
Follow-up
Patients were interviewed by the researchers, either at the clinics or in their homes, at least once a month. Routine blood, urine, and liver and renal function tests, ECG monitoring, and a morphine urine test were done once a month. Before the study, all the researchers carried out the consistency test for the rating scales of adverse effects, euphoria, and other effects (k = 0).
Assessments included the retention rates of naltrexone in both the double-blind study and open studies, the euphoric effects of heroin experienced by the patients during the study period, the results of urine tests, and the adverse effects. Analysis was performed using the statistical package SAS 6.40.
Results
Double-blind Study
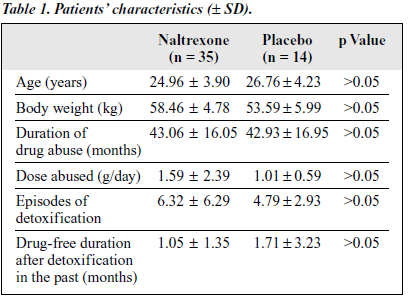
All patients in the double-blind study were referred from 3 detoxif ication centres and had completed detoxif ication without using opiates for at least 5 to 7 days prior to naltrexone treatment. The number of male patients in the naltrexone-treated and placebo groups were 88.57% and 92.86%, respectively. Educational levels below junior high school were 68% and 78.57%, respectively, for the naltrexone-treated and placebo groups, unemployed patients constituted 57.14% and 35.71%, respectively, parents as sponsors were 77.14% and 57.14, respectively, and divorced patients were 20.00% and 21.43%, respectively. Other characteristics are listed in Table 1.
Treatment Efficacy
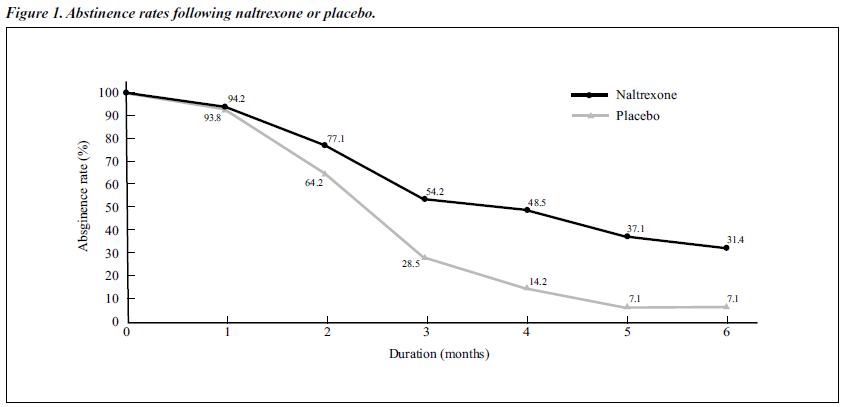
The results of Fisher’s test and the paired t test (after eliminating the influential elements of time by arcsin transformation analysis) showed that there was a significant difference between the abstinence rates of the naltrexone- treated group and the placebo group at different time points from the third to the sixth months (Figure 1).
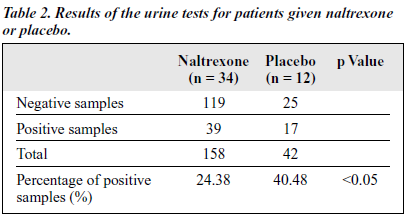
The average abstinence period for the naltrexone group was 3.34 months (SD, ± 2.29 months), which wa s significantly longer than 2.08 months (SD, ± 1.59 months) for the placebo group (p < 0.05). A signif icantly lower percentage of morphine-positive urine tests was found in the naltrexone-treated group (24.38%) than in the placebo group (40.48%) [Table 2].
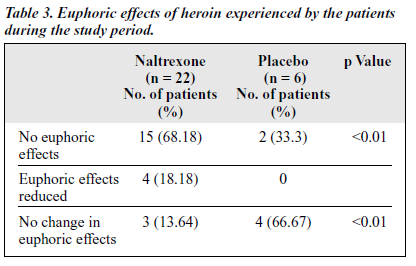
The percentage of patients reporting no euphoria in the naltrexone-treated group was signif icantly higher than in the placebo group, while the number of patients with the same euphoric effects as before the treatment was significantly lower in the naltrexone-treated group than in the placebo group (Table 3).
Adverse Effects
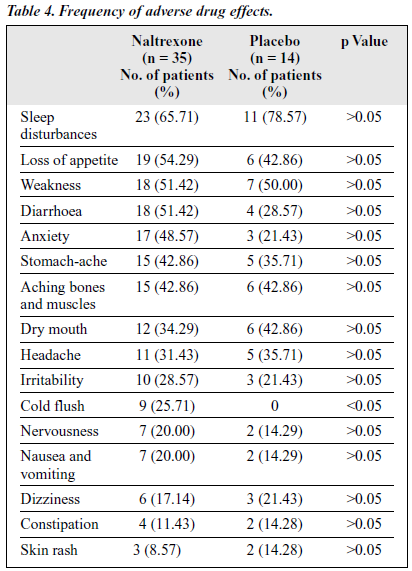
The severity of adverse effects was rated by the researchers during the interview with the patients. The severity of each symptom was scored by different degrees from mild and medium to severe. Mild symptoms meant that they were not the main complaint; medium meant that they were the main complaint but could be endured and treatment was not required; and severe meant that the patient complained about the adverse effects and needed treatment. The adverse effects observed during the course of the study in both the naltrexone-treated and placebo groups are shown in
Although many side effects were recorded, as shown in Table 4, the severity was generally mild and declined during the treatment period. The most common side effects were sleep disturbances (65.71%), loss of appetite (54.29%), weakness (51.42%), and diarrhoea (51.42%). It was assumed that the symptoms and signs of protracted withdrawal syndrome could have contributed to the side effects.
The difference in frequencies of adverse effects between the naltrexone-treated and placebo groups were not statistic- ally significant, suggesting that some of the side effects may have been due to post-withdrawal syndromes. Some changes in laboratory tests were observed in the naltrexone-treated group — notably 5 patients had abnormal glutamic-pyruvic transaminase (GPT) and glutamine-oxaloacetic transaminase
(GOT) results (1 patient had GPT of 547 U/L [normal range, 5 to 31 U/L] and GOT of 231 U/L [normal range, 12 to 28 U/L], the other 4 patients had elevation of either GPT or GOT [<200 U/L]) and 2 patients had bradycardia at ECG monitoring.
Two of the 5 patients with abnormal liver function, including the patient with the most severe episode, reverted to normal after liver function protection treatment, without the need to stop naltrexone treatment. The other 3 patients with abnormal liver function and 2 patients with bradycardia were subsequently lost to follow up.
Open Study
All 267 patients in the open study were referred from 4 detoxification centres and had completed detoxification. The average age of the patients was 27.47 years (SD, ± 7.69 years) and 11.61% were females. The average body weight was 57.86 kg (SD, ± 9.73 kg). The mean duration of opiate abuse and the opiate dosage were 34.84 months (SD, ±18.22 months) and 1.26 g/day (SD, ± 0.89 g/day), respectively. The average number of previous episodes of detoxif ication was 3.76 (SD, ± 2.78) and the previous mean length of abstinence after detoxif ication was 0.5 months (SD, ± 0.63 months).
Of the 267 patients, 28.99% were unemployed, 59.55% had not finished junior high school, 10.49% of the patients’ spouses were opiate abusers, and 8.99% were divorced. For 75.28% patients, their parents were the treatment sponsors.
Treatment Efficacy
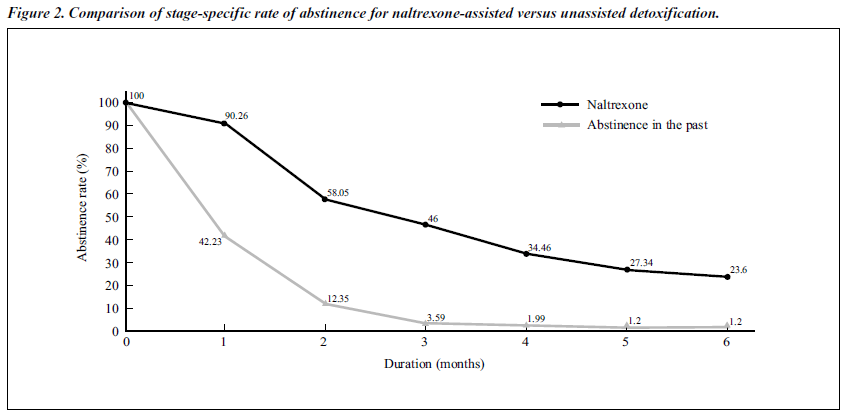
The patients’ previous durations of unassisted abstinence after detoxif ication are reported. Figure 2 shows the comparison of the stage-specif ic rate of abstinence between naltrexone-assisted and previous unassisted abstinence. The retention rate of naltrexone at 6 months was 23.6%, compared with previous abstinence rates of 1.20%.
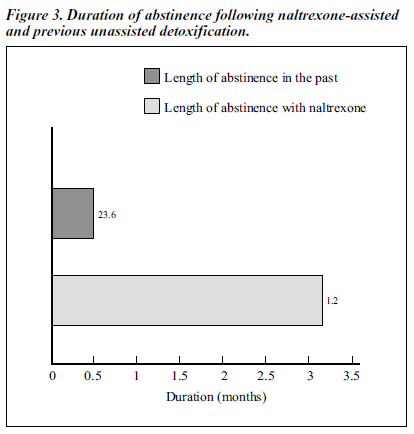
Figure 3 shows that the length of abstinence for naltrexone was 3.16 months (SD, ± 2.29 months), while previous unassisted abstinence was 0.50 months (SD, ± 0.63 months). The difference between length of retention with naltrexone-assisted and previous unassisted abstinence was statistically significant (p < 0.01).
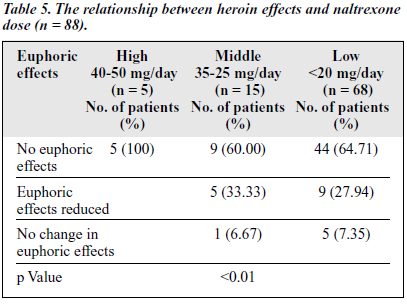
The rate of positive results of the morphine urine test from the first to the sixth months were 14.94%,12.90%, 9.02%, 10.87%, 8.22%, and 7.94%, which indicates a trend of decline of reabuse throughout the course of naltrexone treatment. Table 5 shows that none of the patients in the high-dose group experienced the euphoric effects of heroin, while 6.67% and 7.37% of the patients in the middle and low-dose groups, respectively, experienced euphoric effects.
Table 6 shows that the difference in abstinence rates at different doses of naltrexone were not statistically significant.
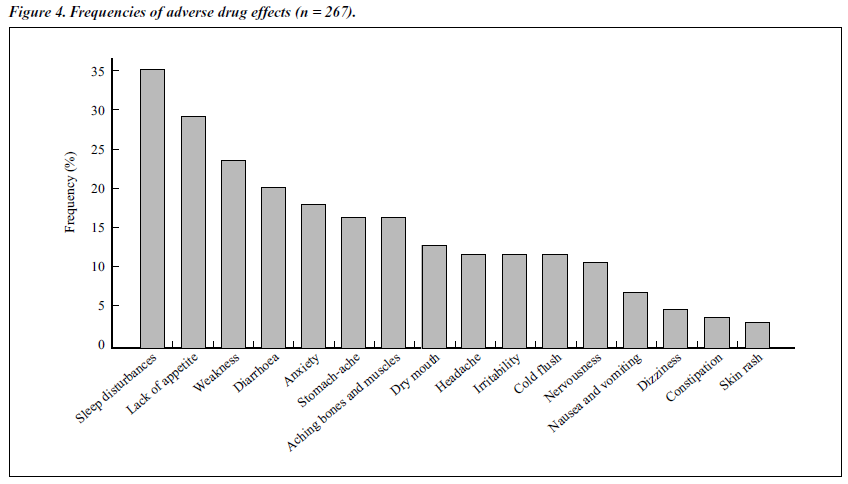
Adverse Effects
The severity of the adverse effects was the same as for the double-blind trial. Figure 4 shows the frequencies of adverse drug effects in the open study group. Similar to those observed in the double-blind study, many side effects were noted, but the severity was mild. As the treatment of naltrexone continued, the severity declined. It is possible that the symptoms and signs of protracted withdrawal syndrome contributed to the effects.
Discussion
There is a high relapse rate for heroin addiction after detoxification. Naltrexone, a competitive long-acting opiate receptor antagonist, may reduce positive reinforcement of the effects of heroin and, in turn, minimise craving. This drug has the ability to prevent reabuse of opiates.1-4
In this study, the eff icacy of naltrexone was assessed by the abstinence rate, the rate of positive urine screening tests, and the euphoric effects of heroin experienced by the patients during treatment with naltrexone. The optimum dosage and the adverse effects were assessed among Chinese heroin addicts.
Treatment Efficacy
In the double-blind study, the difference in abstinence rate between the naltrexone-treated and placebo groups was statistically signif icant. The abstinence rates of the naltrexone-treated group from the third to the sixth month were 54.29%, 48.57%, 37.14%, and 28.57%, which were significantly higher than those of the placebo group (28.57%, 14.29%, 7.14%, and 7.14%). The mean duration of treatment with naltrexone (3.34 months) was significantly higher than that for placebo (2.08 months).
In the open study, the abstinence rates for the naltrexone- treated group from the first to the sixth month were higher than the previously recorded abstinence rates. The compari- son of stage-specif ic rates of abstinence for naltrexone- assisted versus previously unassisted abstinence was statistically significant. The comparison between duration of abstinence of naltrexone-assisted detoxif ication (3.16 months) and previously unassisted detoxif ication (0.50 months) was also statistically significant (p value).
Regarding the euphoric effects of heroin experienced during the naltrexone treatment period in the double-blind study, 68.18% of patients in the naltrexone-treated group had no euphoric effects compared with 33.3% in placebo group — a statistically signif icant result.
The positive urine test rate was 24.38% in the naltrexone- treated group compared with 40.48% in the placebo group — the difference between the 2 groups was statistically signif icant. In the open study, the morphine urine tests indicated that reabuse declined as the naltrexone treatment continued. The above results indicate that by blocking the euphoric effects of heroin and reducing craving, naltrexone maintenance treatment could be used to prevent relapse after successful detoxif ication.
Adverse Effects
The adverse effects observed in both the double-blind and open studies were generally mild and the symptom frequencies remained <40%. Except for the symptom of cold flush and the abnormal GPT and GOT results, the difference in adverse effects between the 2 groups in the double-blind study were not signif icant and most of the side effects disappeared after 3 months of treatment. These results indicate that the adverse effects may be partly due to protracted symptoms that are frequently experienced by heroin addicts after detoxif ication.
Except for the 4 patients with abnormal ECG results (1 patient with ventricular premature beats, 2 with bradycardia, and 1 with high electric potential in the right ventricle), there were no severe adverse effects involving heart function. No abnormal routine urine or blood results were found. These results indicate that naltrexone is a safe drug for maintenance treatment. However, ECG and liver function should be monitored at 1- to 2-month intervals to detect possible transient elevation of transaminases or abnormal ECG.
Optimal Dosage
The dose of naltrexone given in the double-blind study was 50 mg/day according to the international literature. To investigate the optimal dose for Chinese addicts, naltrexone was titrated by the researchers according to the response of the patients.
During the naltrexone treatment, some patients refused to take the higher dose because of the expense of medication and possible side effects. However, the adverse effects were generally mild in both studies. Although there was no difference in the abstinence rates between the different doses (Table 5), the higher dose of naltrexone decreased the euphoric effect more eff iciently than the medium or low dose. Naltrexone 40 to 50 mg/day is still recommended for maintenance treatment for Chinese addicts with the aim of blocking the pharmacological effects of heroin, including craving.
The naloxone challenge and titration procedure is still required since withdrawal symptoms may still be precipitated by naltrexone 50 mg, even after the naloxone challenge test, because naltrexone possesses a stronger antagonistic effect than naloxone. Generally, 3 to 5 days are needed to tolerate naltrexone.
Factors Influencing Naltrexone Treatment
Compliance is a crucial factor of treatment with naltrexone. Whenever naltrexone is taken, patients cannot experience the effects of opiates. This why only a few patients accept naltrexone treatment after detoxification. In our study, some negative factors impacted on the continuation of naltrexone maintenance treatment, including peer-group influence, insuff icient understanding of the role of naltrexone in maintaining abstinence, negative events in daily life, or the expense of naltrexone treatment.
The patients who succeeded were those who were highly motivated and had f irm support from both their family and the community. The psychological and behavioural treatment, as well as regular care by medical and social workers may promote compliance and result in maximising the effects of naltrexone on relapse prevention.
Conclusion
The results of this study of 302 heroin addicts receiving naltrexone treatment suggest that naltrexone is a safe and effective adjunct medication for the prevention of relapse of heroin dependants. The optimum dose of naltrexone is 40 to 50 mg daily. Naltrexone may benefit patients who have strong intentions to abstain from opiates and have suff icient support from their family and society.
References
- Jiang Z. Heroin addiction and contemporary treatment. 1st ed. Beijing: Beijing Scientific Publishing House; 1995:205-218.
- Jiang Z. The clinical application and evaluation of opiate-receptor antagonist: Naltrexone (review part 1). Chinese Mag Drug Abuse Prevent Treat 1995;3:17-18.
- Jiang Z. The clinical application and evaluation of opiate-receptor antagonist: naltrexone (review part 2). Chinese Mag Drug Abuse Prevent Treat 1996;1:35-37.
- Wang X, Qin B. The influence of naltrexone to morphine and heroin addiction. Chin Bull Drug Dep 1994;3(2):74-79.
- National Research Council Committee on Clinical Evaluation of Narcotic Antagonists. Clinical evaluation of naltrexone treatment of opiate-dependent individuals. Arch Gen Psychiatry 1978;353:335-340.
- Mello NK, Mendelson JH, Kuehnle JC, Sellers MS. Operant analysis of human heroin self-administration and the effects of naltrexone. J Pharmacol Exp Ther 1981;216:45-54.
- San L, Pomarol G, Peri JM, Olle JM, Cami J. Follow-up after a six- month maintenance period on naltrexone versus placebo in heroin addicts. Br J Addict 1991:86:983-990.
- Shufman EN, Porat S, Witztum E, Gandacu D, Hamburger RB, Ginath Y. The efficacy of naltrexone in preventing reabuse of heroin after detoxif Biol Psychiatry 1994;35:935-945.
- Kleber HD. Naltrexone. J Subs Abuse Treat 1985;2:117-122.