Hong Kong J Psychiatry 2003;13(3):17-22
ORIGINAL ARTICLE
Abstract
Objective: A subcategory of depression in elderly people, termed vascular depression, has previously been proposed. This study tests one hypothesis of this proposal, the association between the presence of white matter hyperintensities seen on magnetic resonance imaging and clinical symptoms characteristic of vascular depression.
Patients and Methods: Ten consecutive patients referred to an old age psychiatry unit, older than 65 years and meeting the International Classification of Diseases-10 criteria for depression, were screened for symptoms of vascular depression. Clinical ratings of ‘classical’ depressive symptoms and physical morbidity were also completed. The intensity of white matter hyperintensities on magnetic resonance imaging for each patient was blindly rated by a consultant radiologist.
Results: The severity of vascular depression symptoms was positively correlated with the severity of white matter hyperintensities. White matter hyperintensity severity was not correlated with age, gender, or physical morbidity. The mean severity of white matter hyperintensities was significantly greater in the vascular depression subgroup compared with the ‘classical’ depression subgroup.
Conclusions: A subcategory of depression in the elderly, vascular depression, is associated with increased severity of white matter hyperintensities seen on magnetic resonance imaging. This subcategory can be defined purely on the grounds of clinical symptoms.
Key words: Depression, Pathology, Vascular factors
Richard Tranter, MB ChB, MRCPsych, Locum Consultant Psychiatrist, North West Wales NHS Trust, Bangor, North Wales, UK.
Manikkarasa Devakumar, MB ChB, MRCPsych, MD, North West Wales NHS Trust, Bangor, North Wales, UK.
Caroline Adams, MB ChB, FRCR, North West Wales NHS Trust, Bangor, North Wales, UK.
Address for correspondence: Richard Tranter, Consultant Psychiatrist, Hergest Unit, North West Wales NHS Trust, Bangor, Gwynedd, LL57 2PW, UK.
Tel: (44 1248) 384 452
E-mail: Richard.Tranter@nww-tr.wales.nhs.uk
Submitted: 2 May 2003; Accepted: 18 August 2003
Introduction
It has been nearly a quarter of a century since Robinson et al demonstrated a possible association between vascular pathology in the brain and depression using both animal models1 and case studies.2 Although Robinson et al’s original proposition, that left hemisphere strokes cause depression, has not stood the test of time,3 interest in the concept of ‘vascular depression’ has grown.
The association has been demonstrated in both directions, with depressed outpatients appearing to show higher rates of hypertension,4 while patients suffering coronary artery disease show higher rates of depression.5 Baldwin has suggested that late-onset depression in particular is associated with vascular risk factors and vascular disease.6 This subgroup of patients have less genetic susceptibility to depression,7 and would also be expected to have greater psychological resilience, which focuses interest on possible neuropathological aetiologies.
The advent of magnetic resonance imaging (MRI) and the description of white matter hyperintensities (WMH), or leukoaraiosis, by Hachinski et al8 has fuelled a contemporary debate as to whether late-onset depression is associated with vascular pathology in the brain.6 Depressed elderly patients have been shown to have more WMH than non-depressed elderly patients.9 Late-onset depression may also be associated with greater subcortical hyperintensities in the basal ganglia compared with early-onset depression.10 Harrel et al found that those depressed patients with the most severe WMH also had significantly lower mini-mental state examination (MMSE) scores compared with those depressed patients with mild WMH.11 In a review of neuroimaging studies in depression, Soares and Mann have suggested the following clinical correlations to subcortical WMHs: apathy, psychomotor slowing, and poorer response to antidepressant treatment.12
Some authors have elaborated on these observations to suggest that a subset of depression in the elderly, termed ‘vascular depression’, is pathologically related to the presence of WMHs. Krishnan et al have put forward MRI- defined criteria for diagnosing vascular depression,13 which include a score greater than 2 using the criteria of Fazekas et al,14 which provides an assessment of the extent of subcortical grey matter, deep white matter, and peri- ventricular changes on brain MRI.
Alexopolous et al have suggested a clinical definition.15 This includes a history of late-onset depression, or a previous history of depression, the presentation of which has changed in later life. There is impairment of cognitive functions, particularly executive functions, and lack of insight. Psychomotor retardation and disability are prominent, but typical depressive cognitions (such as guilt or worthlessness) are characteristically lacking. There tends to be an absence of a family history of mood disorders compared with early- onset depression. Patients with vascular depression may follow a more chronic course, respond less well to somatic treatments, and show greater cognitive decline compared with those patients with ‘classical’ depression.16
One theory for the aetiology of this presentation of depression is that WMH represents microvascular disease.17 Damage of the blood supply to subcortical striato-pallido- thalamo-cortical pathways could provoke demyelination, disrupting neurotransmitter circuitry involved in mood regulation, and hence predispose to depression.18
The evidence supporting a vascular depression hypo- thesis is circumstantial and open to challenge. Despite repeated demonstrations of the association between vascular disease and WMH, their pathological status has not been characterised. While Thomas et al recently showed a relatively specific association between late-life depression and postmortem atherosclerotic cerebrovascular disease, they could not demonstrate any direct evidence of microvascular pathology.19 WMH occur in a variety of non- vascular conditions such as hydrocephalus, multiple sclerosis, and Alzheimer’s disease as well as normal ageing.18
The recent report from The Rotterdam Scan Study demonstrates the high prevalence of WMH in the general population, with only 8% of people older than 55 years being completely free of WMH.20 WMH appear to be associated with poorer long-term outcome, not just in unipolar depression, but in bipolar affective disorder as well.21 Finally, Kumar et al argue that the contribution of WMH and cerebrovascular risk factors to late-onset depres- sion become insignificant when the effects of medical illness as a whole and cerebral atrophy are controlled for.22
Perhaps the greatest theoretical uncertainty facing the vascular depression hypothesis is whether the characteristic clinical presentation actually represents an early manifes- tation of dementia. In a longitudinal study, Hickie at al found that WMH severity in late-onset depression significantly predicted progression to vascular dementia.16 WMH predict cognitive decline in the general elderly population,23 and the progression of WMH is correlated to this decline.24
One advantage of the vascular depression hypothesis is that it lends itself to empirical testing. The association between WMH and the proposed clinical features has previously been demonstrated by post hoc analysis. Given that criteria for vascular depression, proposed by Alexopolous et al15 and Krishnan et al13 have also included a history of vascular illness or vascular risk factors, the finding of increased severity of WMH thought to be due to vascular changes raises the criticism of tautology. A more rigorous test of the vascular depression hypothesis would be the comparison of the severity of WMH in 2 subgroups of elderly depressed patients selected according to a priori clinical criteria that do not include assumptions of vascular disease status.
The division of elderly depressed patients into 2 subgroups would use the following clinical criteria: the ‘classical’ depression subgroup would present with sub- jective complaints of low mood, express negative cognitions (worthlessness, guilt, etc) and would have insight into their condition; the vascular depression subgroup would objectively demonstrate psychomotor retardation, lack of motivation, and have less insight.
All patients would then be examined for WMH by brain MRI. The null hypothesis to be tested would be that there was no difference in the severity of WMH between the 2 groups. The vascular depression hypothesis predicts that the subgroup characterised by psychomotor retardation, lack of motivation, and lack of insight would have a greater severity of these brain lesions than the classical depression subgroup. Kumar et al’s assertion that any association between depression and WMH is due to the confounding factor of medical co-morbidity22 would also be examined. Due to the present pilot study involving only a small sample, its power to test other hypotheses, such as the relationship between vascular depression and cognitive functioning, would be limited and, as such, is restricted to testing the single null hypothesis.
Patients and Methods
Patients
The project was conducted at the North West Wales NHS Trust, Bangor, Wales. It was envisaged as a pilot study to assess the feasibility of recruitment, investigation, and definition of subgroups. Ethical approval was obtained from the North Wales Health Authority Ethics Committee (West). Due to funding constraints the sample was limited to 10 consecutive patients referred to the Department of Old Age who met the following criteria:
- older than 65 years
- International Classification of Diseases-10 (ICD-10) diagnosis of a depressive episode
- no history of dementia.
ICD-10 diagnosis was made by a psychiatrist. History of dementia was excluded on the basis of case note review and informant interview. Bilingual information regarding the study was provided and informed consent obtained. The following rating scales were administered at the initial interview:
- Hamilton rating scale for depression (HAM-D)
- Beck’s Depression Inventory (BDI)
- section C (interviewer observations) of the Cambridge Mental Disorders of the Elderly Examination - Revised (CAMDEX-R)
- Cumulative Illness Rating Scale (CIRS)
The BDI was patient-administered and used to measure the negative cognitions associated with classical depression. Section C of the CAMDEX-R is an observer rating of the subject’s behaviour during the interview. The HAM-D is also an objective rating and, for this study, information from carers was also taken into account when completing it. Use of observer and carer information was necessary to enable identification of symptoms when significant loss of insight was evident. Observer rated scales were completed by a psychiatrist.
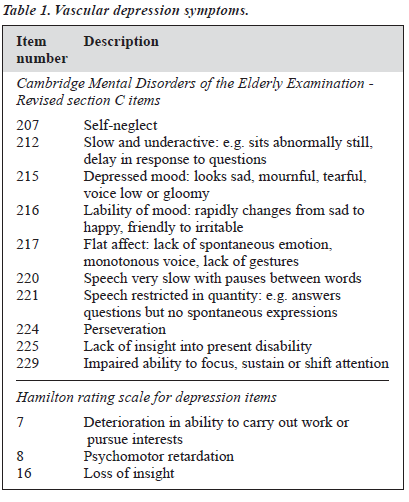
Drawing together criteria from the existing literature, items corresponding to possible symptoms of vascular de- pression were identified in the rating scales used. These items were: 207, 212, 215, 216, 217, 220, 221, 224, 225, and 229 of the CAMDEX-R section C and items 7, 8, and 16 of the HAM-D (Table 1). A vascular depression symptom score was calculated by simply adding together the scores of all these items. A scatter plot of BDI score against vascular depression symptom score for the 10 patients was produced to decide allocation to either of the 2 subcategories of depression. An arbitrary line of division was drawn so that there would be 5 patients in each subgroup. The CIRS was used to quantify overall medical burden, as it was the instrument used by Kumar et al.22 The CIRS has been validated by Linn et al.25
A MRI scan of the brain was then performed for each patient and the films assessed by a consultant radiologist blind to the patient grouping. MRI scans were obtained using a 1.5 Tesla Phillips Gyroscan NT System. Axial images were obtained in a Dual Echo, Turbo Spin Echo sequence (TE 12 ms and 110 ms; TR 3390 ms; slice thickness 5 mm; slice gap 1 mm; 5122 Matrix). Ratings were performed according to the method described by Kumar et al.26 This involves scoring the severity of signal hyperintensities separately on each of the Dual Echo sequences on a scale of 0 to 3 in 3 regions of the brain: periventricular; deep white matter; and basal ganglia and thalamus. If there was a discrepancy between the scores for different Dual Echo sequences the mean was calculated. These scores were added together for each subject to give a composite score of brain MRI WMH.
Analysis
The mean composite WMH scores in the 2 subcategories of depression were compared using an independent t-test. Possible correlations between the CIRS, BDI, vascular depression symptom scores, and WMH scores were also examined using the Spearman rank-order correlation coefficient. All calculations were carried out using the Statistical Package for the Social Sciences.
Results
The mean age of the group was 74.5 years. The scatter plot of BDI scores against vascular depression symptom scores is shown in Figure 1. The results of the correlation analysis are shown in Table 2. The BDI did not demonstrate the
expected negative correlation with WMH scores, and did not aid the division of the sample into 2 subgroups. The vascular depression symptom score was positively correlated with WMH scores, and the 2 subgroups were defined by a simple cutoff for this score. Five patients were allocated to
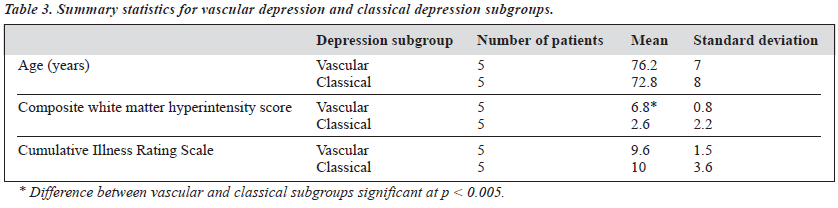
each group on the basis of the arbitrary cutoff: i.e. the 5 patients scoring highest on the vascular depression symptom score were placed in the vascular depression subgroup, the remainder were placed in the classical depression subgroup. Table 3 shows the summary statistics for age, CIRS score, and composite WMH score for the 2 subgroups. The mean score for WMH was significantly greater in the vascular depression subgroup compared to the classical depression subgroup at the level of p < 0.005. The 95% confidence intervals for these means are shown in Figure 2. 10
There was no significant correlation between either the CIRS scores or age and WMH scores (Table 2). There was 8 no significant difference in age or CIRS scores between the 2 comparison subgroups (Table 3). There was no significant difference between the mean WMH scores for men 6 compared with women. A MRI scan from a patient assigned to the classical depression subgroup is shown in Figure 3. The composite WMH score for this individual was 0. By 4 comparison, Figure 4 shows a MRI scan from a patient assigned to the vascular depression subgroup. There are 2 florid signal hyperintensities in the deep white matter and periventricular regions. The composite WMH score for this individual was 8.
Discussion
Depression subgroup (number of patients)
The main limitation of the current study is the sample size. It was intended as a pilot study to examine the feasibility of
recruiting and investigating a larger group. An accurate power calculation of the sample sizes needed in a full scale study was not possible due to the unique group allocation methods employed. From previous studies examining MRI brain changes in depression in general, average sample sizes of 35 patients in depressed and non-depressed groups were used.6 The assumption was that any full scale study would require sample sizes approaching this if the null hypothesis was accepted and a type 2 error avoided at a reasonable level of probability. In the event, the null hypothesis was rejected. The difference observed was greater than expected and, even with the small sample size, resulted in the study having a power of 73%.
The results suggest that a subgroup of depression that is associated with specific MRI changes in the brain can be differentiated purely on the grounds of clinical symptoms. This can be done without specifying the age of onset of depression, the presence or absence of a family history of depression, or the presence or absence of vascular disease or vascular risk factors. More specifically, the current study suggests that the absence of negative cognitions usually present in classical depression (such as thoughts of guilt and worthlessness) as measured by the BDI is less useful as a distinguishing characteristic than the presence of vascular depression symptoms (Table 1).
The present study does not support previous associations between general health status (as measured by the CIRS) and the severity of WMH, although this conclusion is very tentative due to the high risk of a type 2 error with such a small sample.
A future study would look to recruit a larger sample to replicate these results and further refine the clinical characterisation of the vascular depression subgroup. In particular, it would be useful to establish a cutoff for the vascular depression symptom score to aid differentiation of the 2 subgroups. With a larger sample, other factors could also be examined, particularly a comparison of cognitive functioning in the 2 subgroups, and whether the vascular depression subgroup show higher rates of progression to dementia over time. As the clinical and laboratory definition of vascular depression becomes more rigorous, then follow- up studies examining outcome and differential response to treatments can be undertaken. It has already been suggested that interventions aimed at preventing the progression of vascular disease may have a role in the management of vascular depression.27
References
- Robinson RG, Shoemaker WJ, Schlumpf M, Valk T, Bloom FE. Effect of experimental cerebral infarction in rat brain on catecholamines and behaviour. Nature 1975;255:332-334.
- Robinson RG, Kubos KL, Starr LB, Rao K, Price TR. Mood changes in stroke patients: relationship to lesion location. Compr Psychiatry 1983;24:556-566.
- Carson AJ, MacHale S, Allen K, Lawrie SM, Dennis M, House A, Sharpe M. Depression after stroke and lesion location: a systematic review. Lancet 2000;356:122-126.
- Rabkin JC, Charles E, Kass F. Hypertension and DSM III depression in psychiatric outpatients. Am J Psychiatry 1983;140:1072-1074.
- Carney RM, Rich MW, Tevelde A, Saini J, Clark K, Jaffe AS. Major depressive disorder in coronary artery disease. Am J Cardiol 1987;60:1273-1275.
- Baldwin RC. Late life depression and structural brain changes: a review of recent magnetic resonance imaging research. Int J Geriatr Psychiatry 1993;8:115-123.
- Simpson S, Jackson A, Baldwin RC, Burns A. Subcortical hyper- intensities in late-life depression: acute response to treatment and neuropsychological impairment. J Int Psychogeriatr 1997;9:257-275.
- Hachinski VC, Potter P, Merskey H. Leuko-araiosis. Arch Neurol 1987;44:21-23.
- Greenwald BS, Kramer-Ginsberg E, Krishnan RR, Ashtari M, Aupperle PM, Patel M. MRI signal hyperintensities in geriatric depression. Am J Psychiatry 1996;153:1212-1215.
- Iidaka T, Nakajima T, Kawamoto K, et al. Signal hyperintensities on brain magnetic resonance imaging in elderly depressed patients. European Neurol 1996;36:293-299.
- 11. Harrell LE, Duvall E, Folks DG, et al. The relationship of high-intensity signals on magnetic resonance images to cognitive and psychiatric state in Alzheimer’s disease. Arch Neurol 1991;48:1136-1140.
- Soares JC, Mann JJ. The anatomy of mood disorders — review of structural neuroimaging studies. Biological Psychiatry 1997;41:86-106.
- Krishnan KR, Hays JC, Blazer DG. MRI-defined vascular depression. Am J Psychiatry 1997;154:497-501.
- Fazekas F, Offenbacher H, Fuchs S, et al. Criteria for an increased specificity of MRI interpretation in elderly subjects with suspected multiple sclerosis. Neurology 1988;38:1822-1825.
- Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silberweig D, Charlson M. The “vascular depression” hypothesis. Arch Gen Psychiatry 1997;54:915-922.
- Hickie I, Scott E, Wilhelm K, Brodaty H. Subcortical hyperintensities on magnetic resonance imaging in patients with severe depression: a longitudinal evaluation. Biol Psychiatry 1997;42:367-374.
- Lyness JM, Caine ED, King DA, Conwell Y, Olivares T. Cerebrovascular risk factors and later-life depression: testing a small-vessel brain disease model. Am J Geriatr Psychiatry 1998;6:5-13.
- Baldwin R. Aetiology of late-life depression. Adv Psychiatr Treat 1999;5:435-442.
- Thomas AJ, Ferrier IN, Kalaria RN, Perry RH, Brown A, O’Brien JT. A neuropathalogical study of vascular factors in late-life depression. J Neurol Neurosurg Psychiatry 2001;70:83-87.
- de Leeuw FE, de Groot JC, Achten E, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry 2001;70:9-14.
- Moore PB, Shepherd DJ, Eccleston D, et al. Cerebral white matter lesions in bipolar affective disorder: relationship to outcome. Br J Psychiatry 2001;178:172-176.
- Kumar A, Miller D, Ewbank D, et al. Quantitative anatomic measures and comorbid medical illness in late-life major depression. Am J Geriatr Psychiatry 1997;5:15-25.
- Garde E, Mortensen EL, Krabbe K, Rostrupe E, Larsson HB. Relation between age-related decline in intelligence and cerebral white-matter hyperintensities in healthy octogenarians: a longitudinal study. Lancet 2000;356(9230):628-634.
- Schmidt R, Fazekas F, Kapeller P, Schmidt H, Hartung HP. MRI white matter hyperintensities: three-year follow-up of the Austrian stroke prevention study. Neurology 1999;53:132-139.
- Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc 1968;16:622-626.
- Kumar A, Yousem D, Souder E, et al. High-intensity signals in Alzheimer’s disease without cerebrovascular risk factors: a magnetic resonance imaging study. Am J Psychiatry 1992;149:248-250.
- Steffens DC, Krishnan KR. Structural neuroimaging and mood disorders: recent findings, implications for classification, and future directions. Biological Psychiatry 1998;43:705-712.